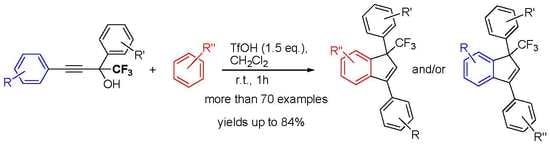
TfOH-Promoted Reaction of 2,4-Diaryl-1,1,1-Trifluorobut-3-yn-2-oles with Arenes: Synthesis of 1,3-Diaryl-1-CF3-Indenes and Versatility of the Reaction Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trost, B.M.; Li, C.J. Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations, 1st ed.; John Wiley & Sons: Weinheim, Germany, 2014. [Google Scholar]
- Stang, P.J.; Diederich, F. Modern Acetylene Chemistry; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Acetylene Chemistry; John Wiley & Sons: Weinheim, Germany, 2005. [Google Scholar]
- Xie, J.; Zhang, T.; Mehrkens, N.; Rudolph, M.; Hashmi, A.S.K. A highly efficient gold-catalyzed photoredoxe α-C(sp3)-H alkynylation of tertiary aliphatic amines with sunlight. Angew. Chem. Int. Ed. 2015, 54, 6046–6050. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Hashmi, A.S.K. Gold-catalyzed reactions of diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. [Google Scholar] [CrossRef] [PubMed]
- Ledovskaya, M.S.; Voronin, V.V.; Rodygin, K.S. Methods for the synthesis of O-, S- and N-vinyl derivatives. Russ. Chem. Rev. 2018, 87, 167–191. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in organic synthesis: Recent progress and new uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef]
- Zakharova, E.A.; Shmatova, O.I.; Nenajdenko, V.G. Acetylene-azide click macrocyclization of peptides. Russ. Chem. Rev. 2018, 87, 619–635. [Google Scholar] [CrossRef]
- Larson, G.L. Some aspects of the chemistry of alkynylsilanes. Synthesis 2018, 50, 2433–2462. [Google Scholar] [CrossRef]
- Lewis, D.E. Intramolecular pericyclic reactions of acetylene derivatives leading to dehydroaromatic and related species. Mini-Rev. Org. Chem. 2017, 14, 107–121. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Schmidt, E.Y. Reactions of acetylenes in superbasic media. Russ. Chem. Rev. 2014, 83, 600–619. [Google Scholar] [CrossRef]
- Begue, J.P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; Published simultaneously in Canada; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tressaud, A.; Haufe, G. (Eds.) Fluorine and Health: Molecular Imaging, Biomedical Materials and Pharmaceuticals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Petrov, V.A. (Ed.) Fluorinated Heterocylic Compounds: Synthesis, Chemistry, and Applications; Published Simultaneously in Canada; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nenajdenko, V.G. (Ed.) Fluorine in Heterocyclic Chemistry; Springer: Berlin, Germany, 2014. [Google Scholar]
- Prakash, R.V. Organofluorine Compounds in Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sasaki, S.; Ikekame, Y.; Tanayama, M.; Yamauchi, T.; Higashiyama, K. Brønsted Acid Catalyzed Friedel-Crafts Alkylation Reactions of Trifluoro-methyl-α,β-ynones with Indoles. Synlett 2012, 23, 2699–2703. [Google Scholar] [CrossRef]
- Kumar, G.G.K.S.N.; Laali, K.K. Condensation of propargylic alcohols with N-methylcarbazole and carbazole in [bmim]PF6 ionic liquid; synthesis of novel dipropargylic carbazoles using TfOH or Bi(NO3)3·5H2O as catalyst. Tetrahedron Lett. 2013, 54, 965–969. [Google Scholar] [CrossRef]
- Sanz, R.; Martinez, A.; Alvarez-Gutierrez, J.M.; Rodriguez, F. Metal-Free Catalytic Nucleophilic Substitution of Propargylic Alcohols. Eur. J. Org. Chem. 2006, 2006, 1383–1386. [Google Scholar] [CrossRef]
- Sanz, R.; Miguel, D.; Martinez, A.; Gohain, M.; Garcia-Garcia, P.; Fernandez-Rodriguez, M.A.; Alvarez, E.; Rodriguez, F. Brønsted Acid Catalyzed Alkylation of Indoles with Tertiary Propargylic Alcohols: Scope and Limitations. Eur. J. Org. Chem. 2010, 2010, 7027–7039. [Google Scholar] [CrossRef] [Green Version]
- Savarimuthu, S.A.; Prakash, D.G.L.; Thomas, S.A. Nucleophilic substitution of propargyl alcohols with aliphatic alcohols, aliphatic amines and heterocycles catalyzed by 4-nitrobenzenesulfonic acid: A scalable and metal-free process. Tetrahedron Lett. 2014, 55, 3213–3217. [Google Scholar] [CrossRef]
- Gujarathi, S.; Hendrickson, H.P.; Zheng, G. Amberlite IR-120H as an efficient and versatile solid phase catalyst for nucleophilic substitution of propargylic alcohols. Tetrahedron Lett. 2013, 54, 3550–3553. [Google Scholar] [CrossRef]
- Srihari, P.; Reddy, J.S.S.; Mandal, S.S.; Satyanarayana, K.; Yadan, J.S. PMA-Silica Gel Catalyzed Propargylation of Aromatic Compounds with Arylpropargyl Alcohols under Solvent-Free Conditions. Synthesis 2008, 12, 1853–1860. [Google Scholar] [CrossRef]
- Zhan, Z.P.; Yu, J.L.; Liu, H.J.; Cui, Y.Y.; Yang, R.F.; Yang, W.Z.; Li, J.P. A General and Efficient FeCl3-Catalyzed Nucleophilic Substitution of Propargylic Alcohols. J. Org. Chem. 2006, 71, 8298–8301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Muth, E.; Flӧrke, U.; Henkel, G.; Merz, K.; Sauvageau, J.; Schwake, E.; Dyker, G. Alkylation of Arenes with Benzylic and Propargylic Alcohols—Classical versus Fancy Catalysts. Adv. Synth. Catal. 2006, 348, 456–462. [Google Scholar] [CrossRef]
- Georgy, M.; Boucard, V.; Campagne, J.M. Gold(III)-Catalyzed Nucleophilic Substitution of Propargylic Alcohols. J. Am. Chem. Soc. 2005, 127, 14180–14181. [Google Scholar] [CrossRef] [PubMed]
- Yadan, J.S.; Reddy, B.V.S.; Rao, K.V.R.; Narayana Kumar, G.G.K.S. Indium(III) Bromide Catalyzed Rapid Propargylation of Heteroaromatic Systems by α-Aryl-Substituted Propargyl Alcohols. Synthesis 2007, 20, 3205–3210. [Google Scholar]
- Masuyama, Y.; Hayashi, M.; Suzuki, N. SnCl2-Catalyzed Propargylic Substitution of Propargylic Alcohols with Carbon and Nitrogen Nucleophiles. Eur. J. Org. Chem. 2013, 2013, 2914–2921. [Google Scholar] [CrossRef]
- Zhan, Z.P.; Yang, W.F.; Yu, J.L.; Li, J.P.; Liu, H.J. BiCl3-Catalyzed propargylic substitution reaction of propargylic alcohols with C-, O-, S- and N-centered nucleophiles. Chem. Commun. 2006, 31, 3352–3354. [Google Scholar] [CrossRef] [PubMed]
- Gohain, M.; Marais, C.; Bezuidenhoudt, B.C.B. Al(OTf)3: An efficient recyclable catalyst for direct nucleophilic substitution of the hydroxy group of propargylic alcohols with carbon- and heteroatom-centered nucleophiles to construct C-C, C-O, C-N and C-S bonds. Tetrahedron Lett. 2012, 53, 1048–1050. [Google Scholar] [CrossRef]
- Gohain, M.; Marais, C.; Bezuidenhoudt, B.C.B. An Al(OTf)3-catalyzed environmentally benign process for the propargylation of indoles. Tetrahedron Lett. 2012, 53, 4704–4707. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Rao, K.V.R.; Narayana Kumar, G.G.K.S.N. Sc(OTf)3-catalyzed alkylation of indoles with propargyl alcohols: An expeditious synthesis of 3-substituted indoles. Tetrahedron Lett. 2007, 48, 5573–5576. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Yin, G.; Lu, P.; Wang, Y. 3-Alkenylation or 3-Alkylation of Indole with Propargylic Alcohols: Construction of 3,4-Dihydrocyclopenta[b]indole and 1,4-Dihydrocyclopenta[b]indole in the Presence of Different Catalysts. J. Org. Chem. 2012, 77, 9510–9520. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.C.; Mendes, S.R.; Martins, G.M. Propargylation of aromatic compounds using Ce(OTf)3 as catalyst. Tetrahedron Lett. 2012, 53, 1567–1570. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Schwarz, L.; Rubenbauer, P.; M. Blanco, C. The Condensation of Carbonyl Compounds with Electron-Rich Arenes: Mercury, Thallium, Gold or a Proton? Adv. Synth. Catal. 2006, 348, 705–708. [Google Scholar] [CrossRef]
- Vasilyev, A.V. Superelectrophilic activation of alkynes, alkenes, and allenes. Adv. Org. Synth. 2018, 8, 81–120. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Giri, S.; Duley, S. Update 2 of: Electrophilicity Index. Chem. Rev. 2011, 111, 43–75. [Google Scholar] [CrossRef]
- Radix-Large, S.; Kucharski, S.; Langlois, B.R. Trifluoromethylated Vinylic and Aromatic Compounds from α-(Trifluoromethyl)allyl Alcohols. Synthesis 2004, 2004, 456–465. [Google Scholar]
- Boreux, A.; Lonca, G.H.; Riant, O.; Gagosz, F. Synthesis of Trifluoromethyl-allenes by Gold-Catalyzed Rearrangement of Propargyl Benzyl Ethers. Org. Lett. 2016, 18, 5162–5165. [Google Scholar] [CrossRef] [PubMed]
- Ghavtadze, N.; Roland, F.; Wuerthwein, E.U. Acid-Mediated Electrocyclic Domino Transformations of 5,5-Disubstituted 1-Amino-1-azapenta-1,4-dien-3-ones into Dihydrospiroindenepyrazole and Dihydroindenodiazepine Derivatives. J. Org. Chem. 2009, 74, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.D.; Fujio, M.; Mohammed, N.; Tidwell, T.; Tsuji, Y. 3-(Trifluoromethyl)indenyl Cation: Ion Pair Return in the Formation of an Antiaromatic and Electron-Deficient Doubly Destabilized Carbocation. J. Org. Chem. 1997, 62, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Gassman, P.G.; Ray, J.A.; Wenthold, P.G.; Mickelson, J.W. Synthesis of perfluoroalkylated indenes. J. Org. Chem. 1991, 56, 5143–5146. [Google Scholar] [CrossRef]
- Martynov, M.Y.; Iakovenko, R.O.; Kazakova, A.N.; Boyarskaya, I.A.; Vasilyev, A.V. Acid-promoted cyclization of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-oles and their TMS-ethers into CF3-indenes. Org. Biomol. Chem. 2017, 12, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |






| Caption | EHOMO, eV | ELUMO, eV | ω a, eV | q(C2) b, e | q(C4) b, e | k(C2)LUMO c, % | k(C4)LUMO c, % |
|---|---|---|---|---|---|---|---|
 | −7.40 | −3.57 | 3.92 | 1.00 | −0.34 | 13.2 | 9.4 |
 | −7.69 | −5.03 | 7.59 | 0.043 | 0.23 | 28.5 | 19.9 |

| Entry | Reaction Conditions a | Yield of 4aa, b % | ||
|---|---|---|---|---|
| Acid | Temperature, °C | Time, h | ||
| 1 | TfOH (50 eq.) | r.t. | 1 | 30 |
| 2 | TfOH (50 eq.) c | −35 | 1 | 45 |
| 3 | TfOH (1.5 eq.) | r.t. | 1 | 57 |
| 4 | FSO3H (86 eq.) c | −75 | 1 | 44 |
| 5 | H2SO4 (5 eq.) | r.t. | 1 | 40 |
| 6 | AlCl3 (2 eq.) | r.t. | 1 | 33 |
| 7 | FeCl3 (1eq.) | r.t. | 1 | 40 |
| 8 | BF3 × Et2O (2 eq.) | r.t. | 72 | 27 d |
| 9 | Sc(OTf)3 (0.1 eq.) e | 85 | 1 | 42 |
| 10 | Cu(OTf)2 (0.1 eq.) e | 85 | 1 | 30 |

| Entry | Alcohol | Reaction Products 4a, 5a, and 6 (Yield, %, Ratio of Isomers) | Possible Reaction Way and Intermediates from Scheme 1 |
|---|---|---|---|
| 1 |  |  (57%) (57%) | waya: A (or B′), D way b: B″, C′ |
| 2 |  |  (48%) (48%) | wayb: B″, C′ |
| 3 |  |  (69%) (69%) | wayb: B″, C′ |
| 4 |  |  total yield of 47% (4ad:6a:6b = 5:2:1) | 4ad—way b: B″, C′ 6a, 6b—way c: B″, C″ |
| 5 |  |  (73%) (73%) | waya: A (or B′), D way b: B″, C′ |
| 6 |  |  (80%) (80%) | waya: A (or B′), D way b: B″, C′ |
| 7 |  |  (66%) (66%) | waya: A (or B′), D way b: B″, C′ |
| 8 a |  |  (70%) (70%) | waya: A (or B′), D way b: B″, C′ |
| 9 |  |  total yield of 72% (4ai:5aa = 1:3.8) | waya: A (or B′), D way b: B″, C′ |
| 10 |  |  total yield of 47% (4aj:5ab = 1:7) | waya: A (or B′), D way b: B″, C′ |
| 11 |  |  | wayb: B″, C′ |
| 12 |  |  (59%) (59%) | waya: A (or B′), D way b: B″, C′ |
| 13 |  |  (51%) (51%) | waya: A (or B′), D way b: B″, C′ |
| 14 a |  |  (62%) (62%) | waya: A (or B′), D way b: B″, C′ |
| 15 |  | Complex mixture of reaction products | - |
| 16 |  | Complex mixture of reaction products | - |
| 17 |  |  (70%) (70%) | waya: A (or B′), D way b: B″, C′ |
| 18 |  |  (80%) (80%) | waya: A (or B′), D way b: B″, C′ |

| Entry | Alcohol | Reaction Products 4 and 5 (Yield, %, Ratio of Isomers) | Possible Reaction Way and Intermediates from Scheme 1 |
|---|---|---|---|
| 1 |  |  (75%) (75%) | waya: A (or B′), D way b: B″, C′ |
| 2 |  |  (66%) (66%) | wayb: B″, C′ |
| 3 |  |  (44%) (44%) | waya: A (or B′), D way b: B″, C′ |
| 4 |  |  (72%) (72%) | wayb: B″, C′ |
| 5 |  |  (60%) (60%) | waya: A (or B′), D way b: B″, C′ |
| 6 |  |  (74%) (74%) | waya: A (or B′), D way b: B″, C′ |
| 7 |  |  (45%) (45%) | waya: A (or B′), D way b: B″, C′ |
| 8 a |  |  (84%) (84%) | waya: A (or B′), D way b: B″, C′ |
| 9 |  |  (71%) (71%) | waya: A (or B′), D way b: B″, C′ |
| 10 |  |  total yield of 28% (4bj:5ba = 1.5:1)  (34%) (34%) | wayb: B″, C′ |
| 11 |  |  (68%) (68%) | waya: A (or B′), D way b: B″, C′ |
| 12 |  |  (58%) (58%) | waya: A (or B′), D way b: B″, C′ |
| 13 a |  |  (70%) (70%) | waya: A (or B′), D way b: B″, C′ |
| 14 |  |  (59%) (59%) | waya: A (or B′), D way b: B″, C′ |
| 15 |  |  total yield of 25% (4bo:5bc = 1.4:1)  (25%) (25%) | wayb: B″, C′ |
| 16 |  |  (74%) (74%) | waya: A (or B′), D way b: B″, C′ |
| 17 |  |  (78%) (78%) | waya: A, B′, D wayb: B″, C′ |

| Entry | Alcohol | Reaction Products 4 and 5 (Yield, %, Ratio of Isomers) | Possible Reaction Way and Intermediates from Scheme 1 |
|---|---|---|---|
| 1 |  |  total yield of 63% (4aj:5ab = 6:1) | wayb: B″, C′ |
| 2 |  |  total yield of 66% (4ca:5ca = 3:1) | wayb: B″, C′ |
| 3 |  |  total yield of 69% (4cb:5cb = 6:1) | wayb: B″, C′ |
| 4 |  |  total yield of 69% (4cc:5cc = 5.7:1) | wayb: B″, C′ |
| 5 |  |  total yield of 60% (4cd:5cd = 4:1) | wayb: B″, C′ |
| 6 |  |  total yield of 56% (4ce:5ce = 4.9:1) | wayb: B″, C′ |
| 7 |  |  (40%) (40%) | wayb: B″, C′ |
| 8 a |  |  (54%) (54%) | wayb: B″, C′ |
| 9 |  |  total yield of 56% (4ch:5cf = 2.8:1) | wayb: B″, C′ |
| 10 |  |  (63%) (63%) | wayb: B″, C′ |
| 11 |  |  (58%) (58%) | wayb: B″, C′ |
| 12 a |  |  (63%) (63%) | wayb: B″, C′ |
| 13 |  | Complex mixture of reaction products | - |
| 14 |  | Complex mixture of reaction products | - |
| 15 |  |  (64%) (64%) | wayb: B″, C′ |
| 16 |  |  (50%) (50%) | wayb: B″, C′ |

| Entry | Alcohol | Reaction Products 4d and 4e (Yield, %, Ratio of Isomers) | Possible Reaction Way and Intermediates from Scheme 1 |
|---|---|---|---|
| 1 |  |  total yield of 75% (4da:4ea = 11.5:1) | 4da—wayb: B″, C′ 4ea—waya: A (or B′), D |
| 2 |  |  total yield of 69% (4db: 4eb = 11.5:1) | 4db—wayb: B″, C′ 4eb—waya: A (or B′), D |
| 3 |  |  total yield of 50% (4dc:4ec = 13:1) | 4dc—wayb: B″, C′ 4ec—waya: A (or B′), D |
| 4 |  |  total yield of 67% (4dd:4ed = 15.7:1) | 4dd—wayb: B″, C′ 4ed—waya: A (or B′), D |
| 5 |  |  total yield of 66% (4de:4ee = 11.5:1) | 4de—wayb: B″, C′ 4ee—waya: A (or B′), D |
| 6 |  |  total yield of 80% (4df:4ef = 24:1) | 4df—wayb: B″, C′ 4ef—waya: A (or B′), D |
| 7 |  |  total yield of 70% (4dg:4eg = 2.7:1) | 4eg—wayb: B″, C′ 4fg—waya: A (or B′), D |
| 8 a |  |  total yield of 58% (4dh:4eh = 1.8:1) | 4dh—wayb: B″, C′ 4eh—waya: A (or B′), D |
| 9 |  |  total yield of 51% (4di:4ei = 12:1) | 4di—wayb: B″, C′ 4ei—waya: A (or B′), D |
| 10 |  | Complex mixture of reaction products | - |
| 11 |  |  total yield of 54% (4dj:4ej = 11.5:1) | 4dj—wayb: B″, C′ 4ej—waya: A (or B′), D |
| 12 |  |  total yield of 49% (4dk:4ek = 10:1) | 4dk—wayb: B″, C′ 4ek—waya: A (or B′), D |
| 13 |  |  total yield of 66% (4dl:4el = 13:1) | 4dl—way b: B″, C′ 4el—way a: A (or B′), D |
| 14 |  |  total yield of 65% (4dm:4em = 6:1) | 4dm—wayb: B″, C′ 4em—waya: A (or B′), D |
| 15 |  | Complex mixture of reaction products | - |
| 16 |  |  total yield of 49% (4dn:4en = 10:1) | 4dn—wayb: B″, C′ 4en—waya: A (or B′), D |
| 17 |  |  total yield of 57% (4do:4eo = 11.5:1) | 4do—wayb: B″, C′ 4eo—waya: A (or B′), D |

| Entry | Alcohol | Reaction Products 4f and 3 (Yield, %, Ratio of Isomers) | Possible Reaction Way and Intermediates from Scheme 1 |
|---|---|---|---|
| 1 a |  |  total yield of 60% (4fa:3a = 2.8:1) | waya: A (or B′), D |
| 2 |  |  total yield of 49% (4gb:3b = 3.5:1) | waya: A (or B′), D |
| 3 |  |  total yield of 98% (4fc:3c = 4.9:1) | waya: A (or B′), D |
| 4 |  |  total yield of 76% (4fd:3d = 5:1) | waya: A (or B′), D |
| 5 |  |  total yield of 44% (4fe:3e = 6:1) | waya: A (or B′), D |
| 6 |  |  total yield of 70% (4ff:3f = 4:1) | waya: A (or B′), D |
| 7 a |  |  (82%) (82%) | waya: A (or B′), D |
| 8 |  | Complex mixture of reaction products | - |
| 9 |  |  total yield of 54% (4fh:3g = 13:1) | waya: A (or B′), D |
| 10 a |  |  (67%) (67%) | waya: A (or B′), D |
| 11 a |  |  (57%) (57%) | waya: A (or B′), D |
| 12 |  |  total yield of 65% (4fk:3h = 10:1) | waya: A (or B′), D |
| 13 |  |  total yield of 36% (4fl:3i = 7:1) | waya: A (or B′), D |
| 14 |  | Complex mixture of reaction products | - |
| 15 a |  |  (69%) (69%) | waya: A (or B′), D |
| 16 |  | Complex mixture of reaction products | - |
| 17 |  1q 1q |  total yield of 50% (4fn:3j = 2.8:1) | waya: A (or B′), D |
| 18 |  |  total yield of 59% (4fo:3k = 2:1) | waya: A (or B′), D |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerov, A.V.; Kazakova, A.N.; Boyarskaya, I.A.; Panikorovskii, T.L.; Suslonov, V.V.; Khoroshilova, O.V.; Vasilyev, A.V. TfOH-Promoted Reaction of 2,4-Diaryl-1,1,1-Trifluorobut-3-yn-2-oles with Arenes: Synthesis of 1,3-Diaryl-1-CF3-Indenes and Versatility of the Reaction Mechanisms. Molecules 2018, 23, 3079. https://doi.org/10.3390/molecules23123079
Zerov AV, Kazakova AN, Boyarskaya IA, Panikorovskii TL, Suslonov VV, Khoroshilova OV, Vasilyev AV. TfOH-Promoted Reaction of 2,4-Diaryl-1,1,1-Trifluorobut-3-yn-2-oles with Arenes: Synthesis of 1,3-Diaryl-1-CF3-Indenes and Versatility of the Reaction Mechanisms. Molecules. 2018; 23(12):3079. https://doi.org/10.3390/molecules23123079
Chicago/Turabian StyleZerov, Aleksey V., Anna N. Kazakova, Irina A. Boyarskaya, Taras L. Panikorovskii, Vitalii V. Suslonov, Olesya V. Khoroshilova, and Aleksander V. Vasilyev. 2018. "TfOH-Promoted Reaction of 2,4-Diaryl-1,1,1-Trifluorobut-3-yn-2-oles with Arenes: Synthesis of 1,3-Diaryl-1-CF3-Indenes and Versatility of the Reaction Mechanisms" Molecules 23, no. 12: 3079. https://doi.org/10.3390/molecules23123079
APA StyleZerov, A. V., Kazakova, A. N., Boyarskaya, I. A., Panikorovskii, T. L., Suslonov, V. V., Khoroshilova, O. V., & Vasilyev, A. V. (2018). TfOH-Promoted Reaction of 2,4-Diaryl-1,1,1-Trifluorobut-3-yn-2-oles with Arenes: Synthesis of 1,3-Diaryl-1-CF3-Indenes and Versatility of the Reaction Mechanisms. Molecules, 23(12), 3079. https://doi.org/10.3390/molecules23123079