Effects of (S)-Carvone and Gibberellin on Sugar Accumulation in Potatoes during Low Temperature Storage
Abstract
:1. Introduction
2. Results
2.1. Tuber Sprouting
2.2. Physiological Response
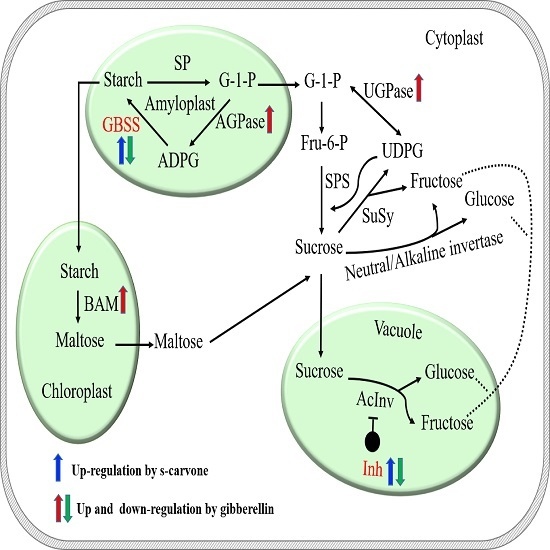
2.3. Sugar and Starch Contents
2.4. Expression Patterns of Starch and Sugar Metabolism Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Sprouting Rate Analysis
4.3. Weight Loss Rate Analysis
4.4. Respiration Rate Analysis
4.5. Sugar and Starch Content Analysis
4.6. RNA Extraction and cDNA Synthesis
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGPase | ADP-glucose pyrophosphorylase |
| Amy | α-Amylase |
| BAM | β-Amylase |
| CIS | Cold-induced sweetening |
| GBSS | Granule-bound starch synthase |
| INH | Invertase inhibitor |
| INV | Vacuolar invertase |
| LSD | Least significant difference |
| qRT-PCR | Quantitative real-time PCR |
| SP | Starch phosphorylase |
| SPS | Sucrose phosphate synthase |
| SuSy | Sucrose synthase |
| UGPase | UDP-glucose pyrophosphorylase |
References
- Struik, P.C.; Wiersema, S.G. Seed Potato Technology; Wageningen University Press: Wageningen, The Netherlands, 1999. [Google Scholar]
- Muthoni, J.; Kabira, J.; Shimelis, H.; Melis, R. Regulation of potato tuber dormancy: A review. Aust. J. Crop Sci. 2014, 8, 754–759. [Google Scholar]
- Aksenova, N.P.; Sergeeva, L.I.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kolachevskaya, O.O.; Romanova, G.A. Regulation of potato tuber dormancy and sprouting. Russ. J. Plant Physiol. 2013, 60, 301–312. [Google Scholar] [CrossRef]
- Chen, X.; Song, B.; Liu, J.; Yang, J.; He, T.; Lin, Y.; Zhang, H.; Xie, C. Modulation of gene expression in cold-induced sweetening resistant potato species Solanum berthaultii exposed to low temperature. Mol. Genet. Genet. 2012, 287, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Muttucumaru, N.; Powers, S.J.; Gillatt, P.N.; Hartley, L.; Elmore, J.S.; Mottram, D.S. Concentrations of free amino acids and sugars in nine potato varieties: Effects of storage and relationship with acrylamide formation. J. Agric. Food Chem. 2012, 60, 12044–12055. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.S.; Garg, N.; Garg, S.; Kumar, A. Starch phosphorylase: Role in starch metabolism and biotechnological applications. Crit. Rev. Biotechnol. 2009, 29, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, S.; Zhao, Y.; Li, B.; Zhang, J. Over-expression of AGPase genes enhances seed weight and starch content in transgenic maize. Planta 2011, 233, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Sun, P.; Liu, W.; Xu, B.; Jin, Z. Identification of genes encoding granule-bound starch synthase involved in amylose metabolism in banana fruit. PLoS ONE 2014, 9, e88077. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sowokinos, J.R.; Hahn, I.S. Regulation of UDP-glucose pyrophosphorylase isozyme UGP5 associated with cold-sweetening resistance in potatoes. J. Plant Physiol. 2008, 165, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Upadhyay, S.K.; Verma, P.C.; Solomon, S.; Singh, S.B. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol. 2011, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Guy, C.L. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2010, 44, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Liu, J.; Ou, Y.; Lin, Y.; Li, M.; Song, B.; Xie, C. A novel RING finger gene, SbRFP1, increases resistance to cold-induced sweetening of potato tubers. FEBS Lett. 2013, 587, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Wiberley-Bradford, A.E.; Busse, J.S.; Bethke, P.C. Temperature-dependent regulation of sugar metabolism in wild-type and low-invertase transgenic chipping potatoes during and after cooling for low-temperature storage. Postharvest Biol. Technol. 2016, 115, 60–71. [Google Scholar] [CrossRef]
- Herman, D.J.; Knowles, L.O.; Knowles, N.R. Low oxygen storage modulates invertase activity to attenuate cold-induced sweetening and loss of process quality in potato (Solanum tuberosum L.). Postharvest Biol. Technol. 2016, 121, 106–117. [Google Scholar] [CrossRef]
- Herman, D.J.; Knowles, L.O.; Knowles, N.R. Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 2016, 245, 1–20. [Google Scholar] [CrossRef]
- Lin, Q.; Xie, Y.; Liu, W.; Zhang, J.; Cheng, S.; Xie, X.; Guan, W.; Wang, Z. UV-C treatment on physiological response of potato (Solanum tuberosum L.) during low temperature storage. J. Food Sci. Technol. 2017, 54, 1–7. [Google Scholar] [CrossRef]
- Foukaraki, S.G.; Cools, K.; Chope, G.A.; Terry, L.A. Impact of ethylene and 1-MCP on sprouting and sugar accumulation in stored potatoes. Postharvest Biol. Technol. 2016, 114, 95–103. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Baldwin, I.T.; Erb, M. Herbivory-induced jasmonates constrain plant sugar accumulation and growth by antagonizing gibberellin signaling and not by promoting secondary metabolite production. New Phytol. 2017, 215, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Gubler, F.; Kalla, R.; Roberts, J.K.; Jacobsen, J.V. Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pl α-amylase gene promoter. Plant Cell 1995, 7, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, G.; Stefano, S.; Marco, L.; Edoardo, N.; Grazia, M.L.C.; Viviana, C.; Stefania, S.; Corrada, G. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Hartmans, K.J.; Scheffer, J.J.C. Inhibition of potato sprout growth by carvone enantiomers and their bioconversion in sprouts. Potato Res. 1995, 38, 219–230. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Jjc, H.K.; Lhw, V. Inhibitory effect of s-carvone on wound healing of potato tuber tissue. Physiol Plantarum. 2010, 93, 225–232. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Leitao, A.C.; Gorris, L.G.M.; Smid, E.J. Comparative study on the action of S-(+)-carvone, in situ, on the potato storage fungi Fusarium solani var. coeruleum and F. sulphureum. J. Appl. Microbiol. 2010, 80, 535–539. [Google Scholar] [CrossRef]
- Sun, T.P.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. J. Plant Growth Regul. 2001, 20, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 2004, 16, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Garcíamartínez, J.L. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J. 2010, 22, 247–256. [Google Scholar] [CrossRef]
- Wiltshire, J.J.J.; Cobb, A.H. A review of the physiology of potato tuber dormancy. Ann. Appl. Biol. 2010, 129, 553–569. [Google Scholar] [CrossRef]
- Huchelmann, A.; Gastaldo, C.; Veinante, M.; Zeng, Y.; Heintz, D.; Tritsch, D.; Schaller, H.; Rohmer, M.; Bach, T.J.; Hemmerlin, A. S-carvone suppresses cellulase-induced capsidiol production in Nicotiana tabacum by interfering with protein isoprenylation. Plant Physiol. 2014, 164. [Google Scholar] [CrossRef] [PubMed]
- Oosterhaven, K.; Hartmans, K.J.; Huizing, H.J. Inhibition of potato (Solanum tuberosum) sprout growth by the monoterpene s-carvone: Reduction of 3-hydroxy-3-methylglutaryl coenzyme a reductase activity without effect on its mrna level. J. Plant Physiol. 1993, 141, 463–469. [Google Scholar] [CrossRef]
- Blenkinsop, R.W.; Copp, L.J.; Yada, R.Y.; Marangoni, A.G. Changes in compositional parameters of potato (Solanum tuberosum) during low-temperature storage and their relationship to chip processing quality. J. Agric. Food Chem. 2002, 50, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, K.S.; Masud, T.; Ali, S.; Khan, S.U.; Mahmood, T.; Qayyum, A. Sugar-starch metabolism and antioxidant potential in potato tubers in response to different antisprouting agents during storage. Potato Res. 2015, 58, 361–375. [Google Scholar] [CrossRef]
- Fauconnier, M.L.; Beltran, J.R.; Delcarte, J.; Dejaegh, F.; Marlier, M.; Jardin, P. Lipoxygenase pathway and membrane permeability and composition during storage of potato tubers (Solanum tuberosum L. cv Bintje and Desiree) in different conditions. Plant Biol. 2002, 4, 77–85. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Karanisa, T.; Akoumianakis, K.; Alexopoulos, A.; Karapanos, I. Effect of postharvest application of carvone on potato tubers grown from true potato seed (TPS). Procedia Environ. Sci. 2015, 29, 166–167. [Google Scholar] [CrossRef]
- Kamrani, M.; Kohnehrouz, B.B.; Gholizadeh, A. Effect of RNAi-mediated gene silencing of starch phosphorylase L and starch-associated R1 on cold-induced sweetening in potato. J. Pomol Hortic Sci. 2016, 91, 625–633. [Google Scholar] [CrossRef]
- Kaplan, F.; Dong, Y.S.; Guy, C.L. Roles of β-amylase and starch breakdown during temperatures stress. Physiol. Plant. 2010, 126, 120–128. [Google Scholar] [CrossRef]
- Peng, T.; Zhu, X.; Duan, N.; Liu, J.H. PtrBAM1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regulon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 2015, 37, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, H.; Liu, J.; Reid, S.; Liu, T.; Xu, S.; Tian, Z.; Sonnewald, U.; Song, B.; Xie, C. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. J. Exp. Bot. 2017, 68, 2317–2331. [Google Scholar] [CrossRef] [PubMed]
- Sowokinos, J.R. Biochemical and molecular control of cold-induced sweetening in potatoes. Am. J. Potato Res. 2001, 78, 221–236. [Google Scholar] [CrossRef]
- Zhou, S.P.; Sauve, R.; Fish, T.; Thannhauser, T.W. Salt-induced and salt-suppressed proteins in tomato leaves. J. Am. Soc. Hortic. Sci. 2009, 134, 289–294. [Google Scholar] [CrossRef]
- Ciereszko, I.; Johansson, H.; Kleczkowski, L.A. Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem. J. 2001, 354, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cheng, S.; Liu, J.; Ou, Y.; Song, B.; Zhang, C.; Lin, Y.; Li, X.Q.; Xie, C. The potato protease inhibitor gene, St-Inh, plays roles in the cold-induced sweetening of potato tubers by modulating invertase activity. Postharvest Biol. Technol. 2013, 86, 265–271. [Google Scholar] [CrossRef]
Sample Availability: Samples of the experimental materials are available from the authors. |








| Reagent | Volume/μL |
|---|---|
| Template | 1 |
| Forward Primer (10 μM) | 0.4 |
| Reverse Primer (10 μM) | 0.4 |
| 2× TransStart® Top Green qPCR SuperMix (+Dye II) | 10 |
| ddH2O | 8.2 |
| Gene Name | Gene ID | Forward Primer | Reverse Primer |
|---|---|---|---|
| EF1a | PGSC0003DMT400014674 | ATTGGAAACGGATATGCTCCA | TCCTTACCTGAACGCCTGTCA |
| SP | PGSC0003DMT400008970 | CAGGAACCAGATGCTGCTCTT | CATAGCCCCATGCTGGGTAGT |
| AGPase | PGSC0003DMT400079823 | GGAGTCCGATTCAATGTGAGAAGAAG | CCAAAACACTCCGGCTAGCATC |
| GBSS | PGSC0003DMT400031568 | TACACAAGAGTGGAACCCAGCGAC | TGTCAACAGGCAAGCCAACTGC |
| UGPase | PGSC0003DMT400034699 | CCATCGAGTTGGGACCTGAA | GGGAATAGACTTGAAACGGCCTAAG |
| SPS | PGSC0003DMT400067951 | TCCACAGGTCGCAAGAGTATCAGG | CCGGATAAAACACTTCGCTCCCAC |
| SuSy | PGSC0003DMT400007506 | TTTGAGGCCTGGTGTCTGGGAATACA | TCCATTCGAGGCTCCGTCGACAA |
| INH1 | PGSC0003DMT400004650 | GCAAGGTTCGGTAGGTATGA | AAACAGTGAGGGTATTGGGT |
| INH2 | PGSC0003DMT400011760 | ACTATCAAAAGTTGCTAAATCC | CCCTTCTTCAAACCTCGTAT |
| INV | PGSC0003DMT400035987 | CAGGGTCTAGCGTGACTGC | TGATGGGACATCGGTGAAA |
| BAM1 | PGSC0003DMT400003933 | TGAGATGCGTGACCATGAGC | CAAGTGGAACTTGCGCTTCC |
| BAM2 | PGSC0003DMT400004686 | AGCACGTATGTTAGCGAAACA | GTTGAACTAAGCCTTCTGGTGA |
| Amy23 | PGSC0003DMT400025601 | GGCATACACAGCCGTTCATCT | ATCCGTCCCCAATCTTCACG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Onik, J.C.; Hu, X.; Duan, Y.; Lin, Q. Effects of (S)-Carvone and Gibberellin on Sugar Accumulation in Potatoes during Low Temperature Storage. Molecules 2018, 23, 3118. https://doi.org/10.3390/molecules23123118
Xie Y, Onik JC, Hu X, Duan Y, Lin Q. Effects of (S)-Carvone and Gibberellin on Sugar Accumulation in Potatoes during Low Temperature Storage. Molecules. 2018; 23(12):3118. https://doi.org/10.3390/molecules23123118
Chicago/Turabian StyleXie, Yajing, Jakaria Chowdhury Onik, Xiaojia Hu, Yuquan Duan, and Qiong Lin. 2018. "Effects of (S)-Carvone and Gibberellin on Sugar Accumulation in Potatoes during Low Temperature Storage" Molecules 23, no. 12: 3118. https://doi.org/10.3390/molecules23123118
APA StyleXie, Y., Onik, J. C., Hu, X., Duan, Y., & Lin, Q. (2018). Effects of (S)-Carvone and Gibberellin on Sugar Accumulation in Potatoes during Low Temperature Storage. Molecules, 23(12), 3118. https://doi.org/10.3390/molecules23123118