Effect of Capsaicin and Other Thermo-TRP Agonists on Thermoregulatory Processes in the American Cockroach
Abstract
:1. Introduction
2. Results
2.1. Behavioral Thermoregulation of Cockroaches Exposed to TRP Ligands
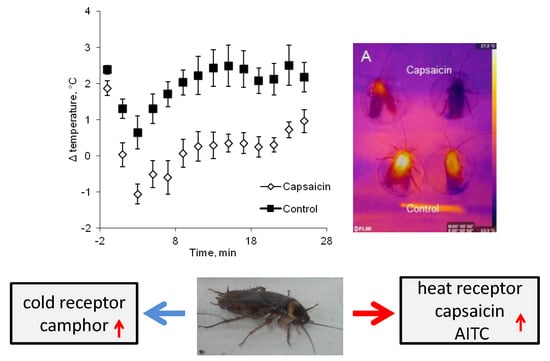
2.2. Insects’ Head Temperature Changes in Response to TRP Agonists and Antagonists
3. Discussion
4. Materials and Methods
4.1. Substances Tested
4.2. Behavioral Assays
4.3. Body Temperature Measurement at Constant Ambient Temperature
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Szolcsányi, J. Capsaicin type pungent agents producing pyrexia. In Pyretics and Antipyretics. Handbook of Experimental Pharmacology; Milton, A.S., Ed.; Springer: Berlin, Germany, 1982; pp. 437–470. ISBN 978-3-642-68571-2. [Google Scholar]
- Szekely, M. Capsaicin-induced changes in behavioural thermoregulation of newborn rabbits (Lepus cuniculus). J. Therm. Biol. 1986, 11, 101–104. [Google Scholar] [CrossRef]
- Kobayashi, A.; Osaka, T.; Namba, Y.; Inoue, S.; Lee, T.H.; Kimura, S. Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am. J. Physiol. 1998, 275, 92–98. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Almeida, M.C.; Garami, A.; Steiner, A.A.; Norman, M.H.; Morrison, S.F.; Nakamura, K.; Burmeister, J.J.; Nucci, T.B. The transient receptor potential vanilloid-1 channel in thermoregulation: A thermosensor it is not. Pharmacol. Rev. 2009, 61, 228–261. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, J.; Tęgowska, E. Capsazepine affects thermal preferences of the American cockroach (Blattodea: Blattidae). Eur. J. Entomol. 2016, 113, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Maliszewska, J.; Tęgowska, E. Capsaicin as an organophosphate synergist against colorado potato beetle (Leptinotarsa decemlineata Say). J. Plant Prot. Res. 2012, 52, 28–34. [Google Scholar] [CrossRef]
- Maliszewska, J.; Marcinkowska, S.; Nowakowska, A.; Kletkiewicz, H.; Rogalska, J. Altered heat nociception in cockroach Periplaneta americana L. exposed to capsaicin. PLoS ONE 2018, 13, e0194109. [Google Scholar] [CrossRef]
- Kallil-Gaspar, P.; Marcuzzo, S.; Rigon, P.; Molina, C.G.; Achaval, M. Capsaicin-induced avoidance behavior in the terrestrial Gastropoda Megalobulimus abbreviatus: Evidence for TRPV-1 signaling and opioid modulation in response to chemical noxious stimuli. Comp. Biochem. Physiol. A 2007, 148, 286–291. [Google Scholar] [CrossRef]
- Summers, T.; Holec, S.; Burrel, B.D. Physiological and behavioral evidence of a capsaicin-sensitive TRPV-like channel in the medicinal leech. J. Exp. Biol. 2014, 217, 4167–4173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, B. Mechanisms of insect thermoregulation. In Effects of Temperature on Ectothermic Organisms; Wieser, W., Ed.; Springer: Berlin, Germany, 1973; pp. 139–150. ISBN 978-3-642-65703-0. [Google Scholar]
- Zars, T. Two thermosensors in Drosophila have different behavioral functions. J. Comp. Physiol. A 2001, 187, 235–242. [Google Scholar] [CrossRef]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008, 454, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nikishawa, M.; Yokohari, F.; Ishibashi, T. Response characteristics of two types of cold receptors on the antennae of the cockroach, Periplaneta americana L. J. Comp. Physiol. A 1992, 171, 299–307. [Google Scholar] [CrossRef]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [Green Version]
- McKemy, D.D. Temperature sensing across species. Pflugers Arch.-Eur. J. Physiol. 2007, 454, 777–791. [Google Scholar] [CrossRef]
- Matsuura, H.; Sokabe, T.; Kohno, K.; Tominaga, M.; Kadowaki, T. Evolutionary conservation and changes in insect TRP channels. BMC Evol. Biol. 2009, 9, 228–239. [Google Scholar] [CrossRef]
- Kohno, K.; Sokabe, T.; Tominaga, M.; Kadowaki, T. Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of transient receptor potential genes. J. Neurosci. 2010, 30, 12219–12229. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Kang, K.J.; Garrity, P.A. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2008, 105, 14668–14673. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.; Shen, W.L.; Shim, H.S.; Montell, C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via TRPV channel in chordotonal neurons. J. Neurosci. 2010, 30, 10465–10471. [Google Scholar] [CrossRef]
- Turner, H.N.; Armengol, K.; Patel, A.A.; Himmel, N.J.; Sullivan, L.; Iyer, S.C.; Bhattacharya, S.; Iyer, E.P.; Landry, C.; Galko, M.J.; et al. The TRP channels Pkd2, NompC, and Trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 2016, 26, 3116–3128. [Google Scholar] [CrossRef]
- Zermoglio, P.F.; Latorre-Estivalis, J.M.; Crespo, J.E.; Lorenzo, M.G.; Lazzari, C.R. Thermosensation and the TRPV channel in Rhodnius prolixus. J. Insect Physiol. 2015, 81, 145–156. [Google Scholar] [CrossRef]
- Olszewska, J.; Tęgowska, E. Opposite effect of capsaicin and capsazepine on behavioral thermoregulation in insects. J. Comp. Physiol. A 2011, 197, 1021–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokabe, T.; Tsujiuchi, S.; Kadowaki, T.; Tominaga, M. Drosophila Painless is a Ca2+-requiring channel activated by noxious heat. J. Neurosci. 2008, 28, 9929–9938. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Tracey, D.W., Jr.; Benzer, S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006, 16, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.P.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnas, M.; Peters, M.; Dadon, D.; Lev, S.; Vertkin, I.; Slutsky, I.; Minke, B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 2009, 45, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.J.; Fu, T.; Yang, T.; Liu, Y.; Wang, G.R. A TRPA1 channel that senses thermal stimulus and irritating chemicals in Helicoverpa armigera. Insect Mol. Biol. 2015, 24, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, H.J.; Germann, T.; Gillen, C.; Hatt, H.; Jostock, R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004, 141, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamkiewicz, B.; Grajpel, B.; Olszewska, J.; Widlińska, O.; Tęgowska, E. The influence of capsaicin application on behavioural thermoregulation in American cockroach Periplaneta americana. In Proceedings of the 17th International Symposium of Polish Network of Molecular and Cellular Biology, Cracow, Poland, 17–19 June 2008; Lach, H., Ed.; Pedagogical University of Cracow: Cracow, Poland, 2008; p. 21. [Google Scholar]
- Minke, B.; Parnas, M. Insight on TRP channels from in vivo studies on Drosophila. Annu. Rev. Physiol. 2006, 68, 649–684. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Trafficking of TRP channel: Determination of channel function. In Transient Receptor Potential (TRP) Channels. Handbook of Experimental Pharmacology Vol. 179; Flockerzi, V., Nilius, B., Eds.; Springer: Berlin, Germany, 2007; pp. 541–557. [Google Scholar]
- Flouris, A.D.; Piantoni, C. Links between thermoregulation and aging in endotherms and ectotherms. Temperature 2015, 2, 73–85. [Google Scholar] [CrossRef]
- Janiszewski, J.; Wysocki, D. Body temperature of cockroaches: Gromphadorhina brauneri (Shelf.) and Periplaneta americana (L.) at high ambient temperatures. Zool. Pol. 1986, 33, 23–32. [Google Scholar]
- Mori, N.; Kawabata, F.; Matsumura, S.; Hosokawa, H.; Kobayashi, S.; Inoue, K.; Fushiki, T. Intragastric administration of allyl isothiocyanate increases carbohydrate oxidation via TRPV1 but not TRPA1 in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1494–1505. [Google Scholar] [CrossRef]
- Gees, M.; Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Everaerts, W.; Segal, A.; Xue, F.; Janssens, A.; Owsianik, G.; Nilius, B.; et al. Mechanisms of transient receptor potential vanilloid 1 activation and sensitization by allyl isothiocyanate. Mol. Pharmacol. 2013, 84, 325–334. [Google Scholar] [CrossRef] [PubMed]
- French, A.S.; Meisner, S.; Liu, H.; Weckström, M.; Torkkeli, P.H. Transcriptome analysis and RNA interference of cockroach phototransduction indicate three opsins and suggests a major role for TRPL channels. Front. Physiol. 2015, 6, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Hew-Butler, T.; Soriano, R.N.; Rao, S.; Wang, W.; Wang, J.; Tamayo, N.; Oliveira, D.L.; Nucci, T.B.; Aryal, P.; et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autononomic and behavioral cold defenses and decreases deep body temperature. J. Neurosci. 2012, 32, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Full, R.J.; Tullis, A. Capacity for sustained terrestrial locomotion in an insect: Energetic, thermal dependence and kinematics. J. Comp. Physiol. B 1990, 160, 573–581. [Google Scholar] [CrossRef]
- Mullins, D.E. Osmoregulation and excretion. In The American Cockroach, 1st ed.; Bell, W.J., Adiyodi, K.G., Eds.; Chapman and Hall: London, UK, 1982; pp. 117–149. ISBN 978-94-009-5827-2. [Google Scholar]
- Kress, H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur. J. Pain 2009, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.W.; Mogensen, T.; Lund, C.; Jacobsen, L.S.; Hjortsoe, N.C.; Rasmussen, S.N.; Rasmussen, M. Analgesic effect of i.v. paracetamol: Possible of paracetamol in postoperative pain. Acta Anaesthesiol. Scand. 2003, 47, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Lo Vecchio, S.; Gazerani, P.; Arendt-Nielsen, L. A dose-response study of topical allyl-isothiocyanate (mustard oil) as human surrogate model of pain, hyperalgesia, and neurogenic inflammation. Pain 2007, 158, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Janiszewski, J.; Tomaszewski, R.; Grygon, B.; Kadziela, W. A simple method for recording body temperature in insect. Acta Physiol. Pol. 1983, 34, 611–616. [Google Scholar]
- Duff, M.; Towey, J. Two ways to measure temperature using thermocouples feature simplicity, accuracy and flexibility. Analog Dialogue 2010, 44, 1–6. [Google Scholar]
Sample Availability: Samples of the compounds: Capsaicin, capsazepine, camphor, menthol, thymol and AITC are available from the authors. |


| Type of Receptor Subfamily | Name | Ligands that Affect Receptors Activity | Literature |
|---|---|---|---|
| Warmth receptors TRPA | Painless (>42 °C) | allyl isothiocyanate (activation) camphor (inhibition) | [15,24,25] |
| Pyrexia (>35 °C) | ? | [15] | |
| dTRPA1 (25–27 °C) | allyl isothiocyanate (activation) | [26] | |
| HsTRPA (Hymenoptera specific TRPA) (>36 °C) | camphor (activation), menthol (inhibition) | [18] | |
| TRPA5 | ? | [17] | |
| Cold receptors | TRP | camphor (activation), thymol and menthol (inhibition) | [27] |
| TRPC | TRPL (10–20 °C) | ||
| TRPP | Brivido 1, −2, −3 (12–15 °C) | ? | [19] |
| TRPV | Inactive (10–20 °C) | ? | [20] |
| NOMPC | Nompc | ? | [21] |
| TRPM | Trpm | ||
| TRPC | Pkd2 (<10 °C) |
| Concentration | Effect | Species | Literature |
|---|---|---|---|
| Camphor | |||
| 3 mM | proboscis extension reflex reduction | honey bee | [18] |
| 4.3 mM (EC50) | AmHsTRPA activation | honey bee | [18] |
| 3 mM | heat-induced activation of Painless inhibition | Drosophila | [24] |
| 10 mM | TRPL activation | Drosophila | [27] |
| 15 mM (repeated doses) | desensitization to heat | Periplaneta americana | [7] |
| Allyl isothiocyanate | |||
| 50 µM | HarmTRPA1 activation | Helicoverpa armigera | [28] |
| 1 mM | AmHsTRPA activation proboscis extension reflex reduction | honey bee | [18] |
| 2 mM | proboscis extension reflex reduction through Painless | Drosophila | [25] |
| 2 mM | proboscis extension reflex reduction through dTRPA1 | Drosophila | [26] |
| Menthol | |||
| 100 nM (EC50) | AmHsTRPA inhibition | honey bee | [18] |
| 0.5 mM | increased fraction of bees in warmer regions | honey bee | [18] |
| 1.8 mM | TRPL inhibition | Drosophila | [27] |
| Thymol | |||
| 1 mM | TRPL inhibition | Drosophila | [27] |
| Capsazepine | |||
| 0.1 µM | preference for warmer regions | Periplaneta americana | [5] |
| 0.1 µM (repeated doses) | desensitization to heat | Periplaneta americana | [7] |
| 0.1 µM 100 µM | preference for warmer regions preference for cooler regions | mealworm Tenebrio molitor larvae | [23] |
| 26 µM | preference for cooler regions (1–1.5 °C); increase in proboscis extension reflex | Rhodnius prolixus | [22] |
| 1 mg/kg | increase in heat-evoked latencies | snail Megalobulimus abbreviatus | [8] |
| 18 µM | block TRPM8 response to menthol | HEK293 cells | [29] |
| Capsaicin | |||
| 0.1 µM | preference for cooler environments | Periplaneta americana | [5] |
| 100 µM | preference for warmer environments | Periplaneta americana | [30] |
| 0.1 µM and 100 µM (repeated doses) | desensitization to heat | Periplaneta americana | [7] |
| 0.1 µM 100 µM | preference for cooler environments | mealworm Tenebrio molitor larvae | [23] |
| 3–12 µM | positive preference for capsaicin, also in painless mutants | Drosophila | [25] |
| 0.34 mM | preference for higher temperatures (2.6 °C); less responsiveness to heat | Rhodnius prolixus | [22] |
| 0.5% | decreased latency for withdrawal behavior from 50 °C | snail Megalobulimus abbreviatus | [8] |
| 0.5 mM | elicited nocifensive behavioral responses | medicinal leech | [9] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliszewska, J.; Jankowska, M.; Kletkiewicz, H.; Stankiewicz, M.; Rogalska, J. Effect of Capsaicin and Other Thermo-TRP Agonists on Thermoregulatory Processes in the American Cockroach. Molecules 2018, 23, 3360. https://doi.org/10.3390/molecules23123360
Maliszewska J, Jankowska M, Kletkiewicz H, Stankiewicz M, Rogalska J. Effect of Capsaicin and Other Thermo-TRP Agonists on Thermoregulatory Processes in the American Cockroach. Molecules. 2018; 23(12):3360. https://doi.org/10.3390/molecules23123360
Chicago/Turabian StyleMaliszewska, Justyna, Milena Jankowska, Hanna Kletkiewicz, Maria Stankiewicz, and Justyna Rogalska. 2018. "Effect of Capsaicin and Other Thermo-TRP Agonists on Thermoregulatory Processes in the American Cockroach" Molecules 23, no. 12: 3360. https://doi.org/10.3390/molecules23123360
APA StyleMaliszewska, J., Jankowska, M., Kletkiewicz, H., Stankiewicz, M., & Rogalska, J. (2018). Effect of Capsaicin and Other Thermo-TRP Agonists on Thermoregulatory Processes in the American Cockroach. Molecules, 23(12), 3360. https://doi.org/10.3390/molecules23123360