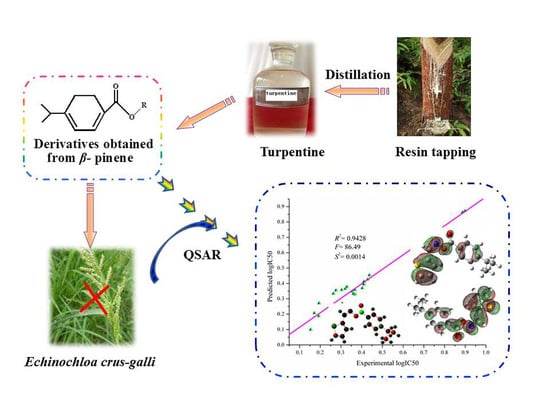
High Add Valued Application of Turpentine in Crop Production through Structural Modification and QSAR Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Derivatives of β-Pinene
2.2. Herbicidal Activity and Structure–Activity Relationships (SARs)
2.3. QSAR Study on Herbicidal Activity Against E. crus-galli
3. Materials and Methods
3.1. Synthesis and Characterizations
3.1.1. Synthesis of 4-Isopropylcyclohexa-1,3-dienecarboxylic acid (Dehydrocumic acid, 3)
3.1.2. Synthesis of 4-Isopropylcyclohexa-1, 3-dienecarboxylates (Compounds 5a–5l)
3.1.3. Synthesis of Oximyl 4-Isopropylcyclohexa-1,3-dienecarboxylates (Compounds 6a–6n)
3.2. Herbicidal Activity Assay of β-Pinene Analogues
3.3. Building and Verification of the Quantitative Structure–Activity Relationship (QSAR) Model
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bangarwa, S.K.; Norsworthy, J.K. Herbicidal activity of three isothiocyanates against yellow nutsedge and their dissipation under two plastic mulches. Crop Prot. 2015, 74, 145–149. [Google Scholar] [CrossRef]
- Ji, Z.Q.; Zhou, F.X.; Wei, S.P. Synthesis and herbicidal activities of benzothiazole N,O-acetals. Bioorganic Med. Chem. Lett. 2015, 25, 4065–4068. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Q.; Li, L.L.; Li, J.; Chen, H.; Song, Z.Q.; Song, J.; Shang, S.B.; Xiao, G.M.; Wang, Z.D.; Rao, X.P. High value-added application of rosin as a potential renewable source for the synthesis of acrylopimaric acid-based botanical herbicides. Ind. Crops Prod. 2015, 78, 131–140. [Google Scholar] [CrossRef]
- Epp, J.B.; Alexander, A.L.; Balko, T.W.; Buysee, A.M.; Brewster, W.K.; Bryan, K.; Daeuble, J.F.; Fields, S.C.; Gast, R.E.; Green, R.A.; et al. The discovery of Arylex TM active and Rinskor TM active: Two novel auxin herbicides. Bioorg. Med. Chem. 2016, 24, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Watkins, D.; Degtyareva, N.N.; Green, K.D.; Spano, M.N.; Garneau-Tsodikova, S.; Arya, D.P. Arginine-linked neomycin B dimers: Synthesis, rRNA binding, and resistance enzyme activity. MedChemComm 2015, 7, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Wang, W.M.; Lu, W.; Niu, C.W.; Li, Y.H.; Li, Z.M.; Wang, J.G. Synthesis and biological evaluation of nonsymmetrical aromaticdisulfides as novel inhibitors of acetohydroxyacid synthase. Bioorg. Med. Chem. Lett. 2013, 23, 3723–3727. [Google Scholar] [CrossRef] [PubMed]
- Mara, C.; Dempsey, E.; Bell, A.; Barlow, J.W. Synthesis and evaluation of phosphor amidate and phosphor othioamidate analogues of amiprophos methyl as potential antimalarial agents. Bioorg. Med. Chem. Lett. 2011, 21, 6180–6183. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Q.; Zhang, J.J.; Liang, X.M.; Zhu, W.J.; Dong, Y.H.; Wu, X.M.; Huang, J.X.; Rui, C.H.; Fan, X.L.; Chen, F.H.; et al. Synthesis and herbicidal activity of 12-(Aryloxyacyloxyimino)-1,15-pentadecanlactone derivatives. J. Agric. Food Chem. 2009, 57, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Sun, H.K.; Cao, H.Y.; Cheng, M.R.; Huang, R.Q. Synthesis and herbicidal activity of 2-cyano-3-substituted-pyridinemethylaminoacrylates. J. Agric. Food Chem. 2003, 51, 5030–5035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Li, H.; Li, Y.H.; Huang, R.Q. Synthesis and herbicidal activity of 2-cyano-3-(2-chlorothiazol-5-yl) methylaminoacrylates. J. Agric. Food Chem. 2004, 52, 1918–1922. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Watkins, D.; Jin, Y.; Gong, C.; King, A.; Washington, A.Z.; Green, K.D.; Garneau-Tsodikova, S.; Adegboyega, K.O.; Arya, D.P. Rapid synthesis, RNA binding, and antibacterial screening of a peptidic-aminosugar (PA) library. ACS Chem. Biol. 2015, 10, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Hossein, H.; Mohammad, J.S.; Mehrdad, N.; Mahmoodreza, M. Natural herbicide activity of Satureja. hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430. [Google Scholar]
- Rachsawan, M.; Warinthorn, C. Antimicrobial, herbicidal and antifeedant activities of mansonone E from the heartwoods of Mansonia gagei Drumm. J. Integr. Agric. 2016, 15, 2795–2802. [Google Scholar]
- Juárez, Z.N.; Hernández, L.R.; Bach, H.; Sánchez-Arreola, E. Antifungal activity of essential oils extracted from Agastachhe Mexicana ssp. xolocotziana and Porophyllumlinariaagainst post-harvest pathogens. Ind. Crops Prod. 2015, 74, 178–182. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Nakano, S.; Shimada, N.; Koumo, C.; Nomiya, K. Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semicarbazone ligands. J. Inorg. Biochem. 2003, 96, 298–310. [Google Scholar] [CrossRef]
- Wang, Z.D.; Song, J.; Chen, J.Z.; Song, Z.Q.; Shang, S.B.; Jiang, Z.K.; Han, Z.J. QSAR study of mosquito repellents from terpeniod with a six-member-ring. Bioorg. Med. Chem. Lett. 2008, 18, 2854–2859. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.Q.; Shang, S.B.; Feng, J.T.; Zhang, X. A value-added use of volatile turpentine: Antifungal activity and QSAR study of b-pinene derivatives against three agricultural fungi. RSC Adv. 2015, 5, 66947–66955. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Tian, X.R.; Li, J.; Shang, S.B.; Song, Z.Q.; Shen, M.G. Study on amphipathic modification and QSAR of volatile turpentine analogues as value-added botanical fungicides against crop-threatening pathogenic fungi. ACS Sustain. Chem. Eng. 2016, 4, 2741–2747. [Google Scholar] [CrossRef]
- Che, J.Y.; Xu, X.Y.; Tang, Z.L.; Gu, Y.C.; Shi, D.Q. Synthesis and herbicidal activity evaluation of novel α-amino phosphonate derivatives containing a uracil moiety. Bioorg. Med. Chem. Lett. 2016, 26, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Robert, D.C. A perspective on the role of quantitative structure-activity and structure-property relationships in herbicide discovery. Pest Manag. Sci. 2012, 68, 513–518. [Google Scholar]
- Prasad, R.K.; Sharma, R. 2D QSAR Analysis of pyrazinecarbox amides derivatives as an herbicidal agent. J. Comput. Methods Mol. Des. 2011, 1, 7–13. [Google Scholar]
- Soung, M.G.; Park, K.Y.; Sung, N.D. 3D-QSAR Study on the influence of alryl amino (R) substituents on herbicidal activity of thiourea analogues. Bull. Korean Chem. Soc. 2010, 31, 1469–1473. [Google Scholar] [CrossRef]
- Zhu, H.L.; Shi, J.; Huang, Z.Q.; Lv, L.J.; Duan, J.W. Structures, specetroscopic analysis, herbicidal activities and enamine-aminone tautomerism of new β-diketone derivatives modified with glycylglycine methyl ester. J. Mol. Struct. 2015, 1089, 170–177. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Li, J.; Shang, S.B.; Wang, D. Synthesis and insecticidal activity of acylthiourea derivatives from β-pinene. Lett. Drug Des. Discov. 2015, 11, 59–66. [Google Scholar]
- Chen, X.B.; Shi, D.Q.; Zhu, X.F. Synthesis, crystal structure and biological activities of O, O-dialkylα-[1-(2-chlorothiazol-5-ylmethyl)-5-methyl-1H-1,2,3-triazol-4-ylcarbonyloxy]alkylphosphonates. Chin. J. Chem. 2007, 25, 1854–1858. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wei, D.G.; Zhu, Y.R.; Liu, S.H.; Zhang, Y.L.; Zhao, Q.Q.; Cai, B.L.; Li, Y.H.; Song, H.B.; Liu, Y.; et al. Synthesis, herbicidal activities, and 3D-QSAR of 2-cyanoacrylates containing aromatic methylamine moieties. J. Agric. Food Chem. 2008, 56, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ugi, I.; Meyr, R.; Lipinski, M.; Bodesheim, F.; Rosendahl, F. Cyclohexyl isocyanide. Org. Synth. 1961, 41, 13–15. [Google Scholar]
- SPSS Inc. SPSS Base Version 17.0 for User’s Guide; SPSS Inc. Press: Chicago, IL, USA, 2008. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02.; Gaussian, Inc. Press: Wallingford, CT, USA, 2004. [Google Scholar]
- Shang, J.; Wang, W.M.; Li, Y.H.; Song, H.B.; Li, Z.M.; Wang, J.G. Synthesis, crystal structure, in vitro acetohydroxy acid synthase inhibition, in vivo herbicidal activity, and 3D-QSAR of new asymmetric aryl disulfides. J. Agric. Food Chem. 2012, 60, 8286–8293. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3, 5a–5l and 6a–6n are available from the authors. |







| Compd. | Herbicidal Activity at a Concentration of (g Active Ingredients/Hectare) | IC50 | y = a + bx | R2 | log IC50 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 100 | 50 | 25 | 12.5 | g Active Ingredients/Hectare | mol Active Ingredients/Hectare | ||||
| 3 | 80 | 68 | 55 | 45 | 32 | 49.880 | 0.300 | y = −0.643 + 0.011x | 0.962 | −0.523 |
| 5a | 100 | 91 | 72 | 57 | 42 | 19.886 | 0.110 | y = −0.617 + 0.031x | 0.991 | −0.959 |
| 5b | 100 | 90 | 71 | 56 | 41 | 21.204 | 0.109 | y = −0.643 + 0.030x | 0.989 | −0.963 |
| 5c | 100 | 90 | 70 | 55 | 41 | 22.024 | 0.106 | y = −0.667 + 0.030x | 0.993 | −0.975 |
| 5d | 100 | 90 | 70 | 54 | 40 | 23.099 | 0.111 | y = −0.712 + 0.031x | 0.993 | −0.955 |
| 5e | 96 | 80 | 68 | 50 | 39 | 25.505 | 0.115 | y = −0.498 + 0.020x | 0.983 | −0.939 |
| 5f | 90 | 78 | 64 | 50 | 39 | 25.679 | 0.116 | y = −0.373 + 0.015x | 0.949 | −0.936 |
| 5g | 100 | 90 | 69 | 53 | 40 | 23.908 | 0.108 | y = −0.736 + 0.031x | 0.996 | −0.967 |
| 5h | 91 | 78 | 65 | 49 | 40 | 25.335 | 0.114 | y = −0.383 + 0.015x | 0.959 | −0.943 |
| 5i | 92 | 81 | 64 | 50 | 38 | 26.605 | 0.113 | y = −0.442 + 0.017x | 0.951 | −0.947 |
| 5j | 88 | 76 | 60 | 50 | 40 | 27.573 | 0.103 | y = −0.360 + 0.013x | 0.959 | −0.987 |
| 5k | 75 | 60 | 49 | 42 | 35 | 64.910 | 0.309 | y = −0.570 + 0.009x | 0.972 | −0.510 |
| 5l | 80 | 60 | 48 | 41 | 35 | 63.125 | 0.249 | y = −0.656 + 0.010x | 0.994 | −0.604 |
| 6a | 100 | 96 | 77 | 61 | 40 | 18.905 | 0.069 | y = −0.768 + 0.041x | 0.990 | −1.161 |
| 6b | 100 | 96 | 76 | 61 | 43 | 17.510 | 0.061 | y = −0.674 + 0.038x | 0.994 | −1.215 |
| 6c | 95 | 84 | 65 | 58 | 37 | 21.708 | 0.068 | y = −0.416 + 0.019x | 0.954 | −1.167 |
| 6d | 93 | 81 | 64 | 55 | 40 | 21.115 | 0.071 | y = −0.345 + 0.016x | 0.965 | −1.149 |
| 6e | 85 | 74 | 59 | 50 | 41 | 26.975 | 0.076 | y = −0.305 + 0.011x | 0.950 | −1.119 |
| 6f | 100 | 95 | 74 | 60 | 40 | 19.723 | 0.073 | y = −0.738 + 0.037x | 0.989 | −1.137 |
| 6g | 90 | 76 | 61 | 54 | 40 | 23.711 | 0.107 | y = −0.323 + 0.014x | 0.967 | −0.971 |
| 6h | 88 | 73 | 60 | 54 | 41 | 23.411 | 0.100 | y = −0.282 + 0.012x | 0.969 | −1.000 |
| 6i | 98 | 85 | 69 | 57 | 40 | 20.229 | 0.075 | y = −0.460 + 0.023x | 0.990 | −1.125 |
| 6j | 93 | 79 | 63 | 55 | 42 | 20.196 | 0.071 | y = −0.314 + 0.016x | 0.982 | −1.149 |
| 6k | 100 | 98 | 78 | 60 | 45 | 17.182 | 0.065 | y = −0.741 + 0.043x | 0.993 | −1.187 |
| 6l | 100 | 97 | 79 | 63 | 44 | 16.004 | 0.065 | y = −0.665 + 0.042x | 0.995 | −1.187 |
| 6m | 100 | 95 | 77 | 60 | 46 | 15.714 | 0.052 | y = −0.564 + 0.036x | 0.998 | −1.284 |
| 6n | 100 | 95 | 75 | 61 | 40 | 19.077 | 0.070 | y = −0.719 + 0.038x | 0.987 | −1.155 |
| Sulfentrazone | 100 | 80 | 69 | 55 | 39 | 15.743 | 0.041 | y = −0.820 + 0.052x | 0.999 | −1.387 |
| Descriptor No. | X | ±ΔX | t-Test | Descriptor |
|---|---|---|---|---|
| 0 | −4.5445 | 7.3386 × 10−1 | −6.1926 | Intercept |
| 1 | −2.5592 × 10−3 | 2.3118 × 10−4 | −11.0701 | ΔHf a |
| 2 | 2.8334 | 3.8752 × 10−1 | 7.3116 | Pµµ b |
| 3 | 7.7801 × 10−2 | 1.0981 × 10−2 | 7.0850 | µ c |
| 4 | −3.4698 × 10−1 | 1.5347 × 10−1 | −2.2609 | qmax° d |
| No. | Compd. | Calc. Log IC50 | Exp. Log IC50 | Difference |
|---|---|---|---|---|
| 1 | 5a | −0.884 | −0.959 | 0.075 |
| 2 | 5b | −0.907 | −0.963 | 0.056 |
| 3 | 5c | −0.926 | −0.975 | 0.049 |
| 4 | 5d | −0.964 | −0.955 | −0.009 |
| 5 | 5e | −0.942 | −0.939 | −0.003 |
| 6 | 5f | −0.952 | −0.936 | −0.016 |
| 7 | 5g | −0.975 | −0.967 | −0.008 |
| 8 | 5h | −0.979 | −0.943 | −0.036 |
| 9 | 5i | −0.949 | −0.947 | −0.002 |
| 10 | 5j | −0.990 | −0.987 | 0.003 |
| 11 | 5k | −0.534 | −0.510 | −0.024 |
| 12 | 5l | −0.628 | −0.604 | −0.024 |
| 13 | 6a | −1.163 | −1.161 | −0.002 |
| 14 | 6b | −1.220 | −1.215 | −0.005 |
| 15 | 6c | −1.023 | −1.167 | 0.044 |
| 16 | 6d | −1.079 | −1.149 | 0.070 |
| 17 | 6e | −1.102 | −1.119 | 0.017 |
| 18 | 6f | −1.067 | −1.137 | 0.070 |
| 19 | 6g | −1.011 | −0.971 | −0.040 |
| 20 | 6h | −1.027 | −1.000 | −0.027 |
| 21 | 6i | −1.183 | −1.125 | −0.058 |
| 22 | 6j | −1.225 | −1.149 | −0.076 |
| 23 | 6k | −1.152 | −1.187 | 0.035 |
| 24 | 6l | −1.109 | −1.187 | 0.078 |
| 25 | 6m | −1.338 | −1.284 | −0.054 |
| 26 | 6n | −1.162 | −1.155 | −0.007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, J.; Li, J.; Song, Z.; Shang, S.; Rao, X. High Add Valued Application of Turpentine in Crop Production through Structural Modification and QSAR Analysis. Molecules 2018, 23, 356. https://doi.org/10.3390/molecules23020356
Gao Y, Li J, Li J, Song Z, Shang S, Rao X. High Add Valued Application of Turpentine in Crop Production through Structural Modification and QSAR Analysis. Molecules. 2018; 23(2):356. https://doi.org/10.3390/molecules23020356
Chicago/Turabian StyleGao, Yanqing, Jingjing Li, Jian Li, Zhanqian Song, Shibin Shang, and Xiaoping Rao. 2018. "High Add Valued Application of Turpentine in Crop Production through Structural Modification and QSAR Analysis" Molecules 23, no. 2: 356. https://doi.org/10.3390/molecules23020356
APA StyleGao, Y., Li, J., Li, J., Song, Z., Shang, S., & Rao, X. (2018). High Add Valued Application of Turpentine in Crop Production through Structural Modification and QSAR Analysis. Molecules, 23(2), 356. https://doi.org/10.3390/molecules23020356