Bauerenol Acetate, the Pentacyclic Triterpenoid from Tabernaemontana longipes, is an Antitrypanosomal Agent
Abstract
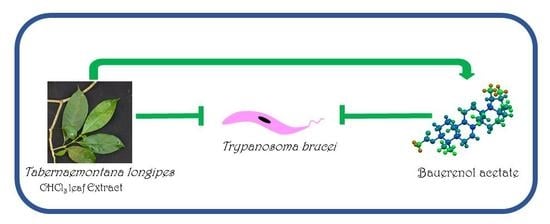
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Compound Characterization
3.2.1. Bauerenol Acetate (1)
3.2.2. Bauerenol (2)
3.3. Trypanosoma brucei Assay
3.4. Cytotoxicity Assay
3.5. Metabolic Profiling
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014, 6, 257–275. [Google Scholar] [PubMed]
- Steverding, D. The history of African trypanosomiasis. Parasites Vectors. 2008, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Lumbala, C.; Simarro, P.P.; Cecchi, G.; Paone, M.; Franco, J.R.; Kande Betu Ku Mesu, V.; Makabuza, J.; Diarra, A.; Chansy, S.; Priotto, G.; et al. Human African trypanosomiasis in the Democratic Republic of the Congo: Disease distribution and risk. Int. J. Health Geogr. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D. Undescribed plants from Guatemala and other Central American republics. Bot. Gaz. 1897, 24, 389–398. [Google Scholar] [CrossRef]
- Morales, J.F. Estudios en las Apocynaceae Neotropicales XV: Sinopsis del género Thoreauea (Apocynoideae, Echiteae), con una nueva especie de Veracruz, México. Brittonia 2005, 57, 258–263. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C NMR spectra of pentacyclic triterpenoids—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Cicció, J.; Hoet, P. Algunos constituyentes de los frutos y delas hojas de Tabernaemontana longipes Donn. Smith. Rev. Latinoam. Quim. 1981, 12, 88–90. [Google Scholar]
- Tinant, B.; Germain, G.; Declercq, J.P.; van Meerssche, M.; Ciccio, J.F.; Hoet, P. Crystal structure of baurenyl acetate from Tabernaemontana longipes Donn. Smith. Bull. Soc. Chim. Belg 2010, 91, 117–121. [Google Scholar] [CrossRef]
- Steverding, D. Evaluation of trypanocidal activity of combinations of anti-sleeping sickness drugs with cysteine protease inhibitors. Exp. Parasitol. 2015, 151, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Nwaka, S.; Hudson, A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.A.; Andrade e Silva, M.L.; Crotti, A.E.M.; Cassio Sola Veneziani, R.; Gimenez, V.M.M.; Januário, A.H.; Groppo, M.; Magalhães, L.G.; Dos Santos, F.F.; Albuquerque, S.; et al. Antileishmanial activity of the hydroalcoholic extract of Miconia langsdorffii, isolated compounds, and semi-synthetic derivatives. Molecules 2011, 16, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.P.V.; Oliveira, A.B.; Lombardi, J.A.; Filho, J.D.S.; Chiari, E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol. Pharm. Bull. 2006, 29, 2307–2309. [Google Scholar] [CrossRef] [PubMed]
- Cunha, W.R.; Crevelin, E.J.; Arantes, G.M.; Crotti, A.E.M.; Silva, M.L.A.E.; Furtado, N.A.J.C.; Albuquerque, S.; da Ferreira, D.S. A study of the trypanocidal activity of triterpene acids isolated from Miconia species. Phytother. Res. 2006, 20, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Del Rayo Camacho, M.; Mata, R.; Castaneda, P.; Kirby, G.C.; Warhurst, D.C.; Croft, S.L.; Phillipson, J.D. Bioactive compounds from Celaenodendron mexicanum. Planta Med. 2000, 66, 463–468. [Google Scholar] [CrossRef]
- Cunha, W.R.; Martins, C.; Ferreira, D.S.; Crotti, A.E.M.; Lopes, N.P.; Albuquerque, S. In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med. 2003, 69, 470–472. [Google Scholar] [PubMed]
- Domínguez-Carmona, D.B.; Escalante-Erosa, F.; García-Sosa, K.; Ruiz-Pinell, G.; Gutierrez-Yapu, D.; Chan-Bacab, M.J.; Giménez-Turba, A.; Peña-Rodríguez, L.M. Antiprotozoal activity of Betulinic acid derivatives. Phytomedicine 2010, 17, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Moreira, V.M.; Gonçalves, B.M.F.; Leal, A.S.; Jing, Y. Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat. Prod. Rep. 2012, 29, 1463. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Ogungbe, I.V. In-silico investigation of antitrypanosomal phytochemicals from Nigerian medicinal plants. PLoS Neglect. Trop. D 2012, 6, e1727. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A.; Vivas, J.; Visbal, G.; Contreras, L.M. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by Δ24 (25)-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol. Biochem. Parasitol. 1995, 73, 199–210. [Google Scholar] [CrossRef]
- Urbina, J.A.; Vivas, J.; Lazardi, K.; Molina, J.; Payares, G.; Pirns, M.M.; Piras, R. Antiproliferative effects OF Δ24 (25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: In vitro and in vivo Studies. Chemotherapy 1996, 42, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.I.; Olson, C.L.; Mamede, J.I.; Gazos-Lopes, F.; Epting, C.L.; Almeida, I.C.; Engman, D.M. Sterol targeting drugs reveal life cycle stage-specific differences in trypanosome lipid rafts. Sci. Rep. 2017, 7, 9105. [Google Scholar] [CrossRef] [PubMed]
- Gros, L.; Castillo-Acosta, V.M.; Jiménez, C.J.; Sealey-Cardona, M.; Vargas, S.; Estévez, A.M.; Yardley, V.; Rattray, L.; Croft, S.L.; Ruiz-Perez, L.M.; et al. New azasterols against Trypanosoma brucei: Role of 24-sterol methyltransferase in inhibitor action. Antimicrob. Agents Chemother. 2006, 50, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue® assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; Piccolo, B.D.; Fiehn, O.; Adams, S.H.; Harper, M.E. A novel amino acid and metabolomics signature in mice overexpressing muscle uncoupling protein 3. FASEB J. 2017, 31, 814–827. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| 1 | 2 | |||
|---|---|---|---|---|
| Position | 13C | 1H | 13C | 1H |
| 1 | 36.6 | 1.18, 1.66 (2H, m) | 37.02 | 1.09, 1.64 (2H, m) |
| 2 | 24.3 | 1.66 (2H, m) | 27.8 | 1.61 (2H, m) |
| 3 | 81.3 | 4.51 (1H, dd) | 79.4 | 3.24 (1H, dd) |
| 4 | 37.9 | 39.0 | ||
| 5 | 48.3 | 2.21 (1H, m) | 48.3 | 2.19 (1H, m) |
| 6 | 24.1 | 1.97, 2.14 (2H, m) | 24.3 | 1.97, 2.16 (2H, m) |
| 7 | 116.4 | 5.40 (1H m) | 116.5 | 5.41 (1H m) |
| 8 | 145.6 | 145.4 | ||
| 9 | 50.7 | 1.41 (1H, m) | 50.5 | 1.29 (1H, m) |
| 10 | 35.0 | 35.3 | ||
| 11 | 32.5 | 1.48, 1.56 (2H, m) | 32.5 | 1.48, 1.54 (2H, m) |
| 12 | 31.6 | 1.14, 1.61 (2H, m) | 31.6 | 1.14, 1.60 (2H, m) |
| 13 | 37.8 | 37.86 | ||
| 14 | 37.9 | 37.86 | ||
| 15 | 28.8 | 1.40, 1.50 (2H, m) | 29.0 | 1.41, 1.49 (2H, m) |
| 16 | 16.7 | 1.48, 1.54 (2H, m) | 16.9 | 1.48, 1.54 (2H, m) |
| 17 | 41.2 | 41.3 | ||
| 18 | 55.0 | 1.29 (1H, s) | 55.0 | 1.29 (1H, s) |
| 19 | 35.3 | 1.15 (1H, m) | 35.4 | 1.14 (1H, m) |
| 20 | 31.8 | 1.54 (1H, m) | 31.8 | 1.54 (1H, m) |
| 21 | 29.3 | 1.19, 1.51 (2H, m) | 29.3 | 1.19, 1.51 (2H, m) |
| 22 | 37.8 | 1.18, 1.48 (2H, m) | 37.82 | 1.18, 1.49 (2H, m) |
| 23 | 15.9 | 0.93 (3H, s) | 14.8 | 0.85 (3H, s) |
| 24 | 27.6 | 0.85 (3H, s) | 27.6 | 0.96 (3H, s) |
| 25 | 13.1 | 0.76 (3H, s) | 13.1 | 0.74 (3H, s) |
| 26 | 23.6 | 0.99 (3H, s) | 23.8 | 0.99 (3H, s) |
| 27 | 22.8 | 0.94 (3H, s) | 22.8 | 0.94 (3H, s) |
| 28 | 37.9 | 1.02 (3H, s) | 38.1 | 1.02 (3H, 3) |
| 29 | 25.8 | 1.04 (3H, d) | 25.8 | 1.04 (3H, d) |
| 30 | 22.6 | 0.90 (3H, d) | 22.7 | 0.90 (3H, d) |
| OAc | 21.4, 171.1 | 2.05 (3H, s) | ||
| Compound | T. brucei Growth Inhibition IC50 (µM) | Cytotoxicity Hep G2 IC50 (µM) |
|---|---|---|
| 1 | 3.09 ± 0.80 | >80 |
| 2 | 2.71 ± 0.96 | >80 |
| Suramin | 0.04 ± 0.01 | n/a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carothers, S.; Nyamwihura, R.; Collins, J.; Zhang, H.; Park, H.; Setzer, W.N.; Ogungbe, I.V. Bauerenol Acetate, the Pentacyclic Triterpenoid from Tabernaemontana longipes, is an Antitrypanosomal Agent. Molecules 2018, 23, 355. https://doi.org/10.3390/molecules23020355
Carothers S, Nyamwihura R, Collins J, Zhang H, Park H, Setzer WN, Ogungbe IV. Bauerenol Acetate, the Pentacyclic Triterpenoid from Tabernaemontana longipes, is an Antitrypanosomal Agent. Molecules. 2018; 23(2):355. https://doi.org/10.3390/molecules23020355
Chicago/Turabian StyleCarothers, Simira, Rogers Nyamwihura, Jasmine Collins, Huaisheng Zhang, HaJeung Park, William N. Setzer, and Ifedayo Victor Ogungbe. 2018. "Bauerenol Acetate, the Pentacyclic Triterpenoid from Tabernaemontana longipes, is an Antitrypanosomal Agent" Molecules 23, no. 2: 355. https://doi.org/10.3390/molecules23020355
APA StyleCarothers, S., Nyamwihura, R., Collins, J., Zhang, H., Park, H., Setzer, W. N., & Ogungbe, I. V. (2018). Bauerenol Acetate, the Pentacyclic Triterpenoid from Tabernaemontana longipes, is an Antitrypanosomal Agent. Molecules, 23(2), 355. https://doi.org/10.3390/molecules23020355