Sesquiterpene Lactones from Vernonia cinerascens Sch. Bip. and Their in Vitro Antitrypanosomal Activity
Abstract
:1. Introduction
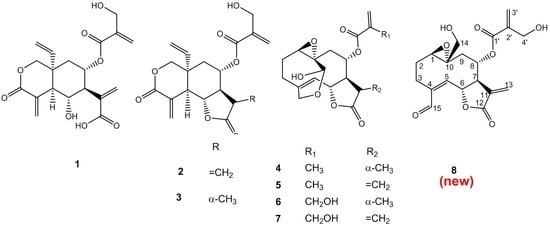
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Analytical Data
3.5. In Vitro Bioassays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO) Neglected Tropical Diseases. Available online: http://www.who.int/neglected_diseases/diseases/summary/en/ (accessed on 13 November 2017).
- World Health Organization Neglected Tropical Diseases. Lowest Caseload Recorded as the World Prepares to Defeat Sleeping Sickness. Available online: http://www.who.int/neglected_diseases/news/HAT_lowest_caseload_recorded/en/ (accessed on 13 November 2017).
- Feasey, N.; Wansbrough-Jones, M.; Mabey, D.C.W.; Solomon, A.W. Neglected tropical diseases. Br. Med. Bull. 2010, 93, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.J.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanosomatid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L. Multi-target-directed ligands in the search for novel lead candidates against Trypanosoma and Leishmania. J. Med. Chem. 2009, 52, 7339–7359. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.S.; Yukich, J.; Goeree, R.; Tediosi, F. A literature review of economic evaluations for a neglected tropical disease: Human African trypanosomiasis (“Sleeping Sickness“). PLoS Negl. Trop. Dis. 2015, 9, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keating, J.; Yukich, J.O.; Sutherland, C.S.; Woods, G.; Tediosi, F. Human African trypanosomiasis prevention, treatment and control costs: A systematic review. Acta Trop. 2015, 150, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V. The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases—Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nugroho, A.E.; Hirasawa, Y.; Nakata, A.; Kaneda, T.; Uchiyama, N.; Goda, Y.; Shirota, O.; Morita, H.; Aisa, H.A. Vernodalidimers A and B, novel orthoester elemanolide dimers from seeds of Vernonia anthelmintica. Tetrahedron Lett. 2010, 51, 6584–6587. [Google Scholar] [CrossRef]
- Herz, W.; Govindan, S.V. Eucannabinolide and other constituents of Schkuhria virgata. Phytochemistry 1980, 19, 1234–1236. [Google Scholar] [CrossRef]
- Romo de vivar, A.; Perez, A.L.C.; Leon, C.; Delgado, G. 11,13-Dehydroeriolin, schkuhrioidin and schkuhriolid, germacranolides from Schkuhria species. Phytochemistry 1982, 21, 2905–2908. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, Y.-L.; Hua, L.; Li, Y.; Hamid-Hussain, S.; Arfan, M.; Gao, K. Guaianolides and elemanolides from Vernonia anthelmintica. Phytochem. Lett. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Zenebe, M.M.; Dessie, K.B.; Hana, G.M.; Werkneh, A.A. Isolation, structural elucidation, and bioactivity studies of leaf extract of Vernonia Amygdalina. Am. J. Appl. Chem. 2015, 3, 14–20. [Google Scholar] [CrossRef]
- Buskuhl, H.; De Oliveira, F.L.; Blind, L.Z.; De Freitas, R.A.; Barison, A.; Campos, F.R.; Corilo, Y.E.; Eberlin, M.N.; Caramori, G.F.; Biavatti, M.W. Sesquiterpene lactones from Vernonia scorpioides and their in vitro cytotoxicity. Phytochemistry 2010, 71, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Anti-trypanosomatid elemanolide sesquiterpene lactones from Vernonia lasiopus O. Hoffm. Molecules 2017, 22, 597. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.T.; Brun, R.; Kaiser, M.; Van Kiem, P.; Van Minh, C.; Schmidt, T.J.; Kang, J.S.; Kim, Y.H. Anti-protozoal activities of cembrane-type diterpenes from Vietnamese soft corals. Molecules 2015, 20, 12459–12468. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J. Structure-activity relationships of sesquiterpene lactones. In Studies in Natural Products Chemistry; ur Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 309–392. [Google Scholar]
- Schmidt, T.J. Helenanolide-type sesquiterpene lactones—III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg. Med. Chem. 1997, 5, 645–653. [Google Scholar] [CrossRef]
- Schmidt, T.J. Toxic activities of sesquitepene lactones: Structural and biochemical aspects. Curr. Org. Chem. 1999, 3, 577–608. [Google Scholar]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative structure—Antiprotozoal activity relationships of sesquiterpene lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Da Costa, F.B.; Lopes, N.P.; Kaiser, M.; Brun, R. In silico prediction and experimental evaluation of furanoheliangolide sesquiterpene lactones as potent agents against Trypanosoma brucei rhodesiense. Antimicrob. Agents Chemother. 2014, 58, 325–332. [Google Scholar]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal sesquiterpene lactones and other oonstituents from Tarchonanthus camphoratus and Schkuhria pinnata. J. Nat. Prod. 2017. [Google Scholar] [CrossRef]
- Vernonia cinerascens in Global Plants on JSTOR. Available online: http://plants.jstor.org/compilation/vernonia.cinerascens (accessed on 3 December 2017).
- Verma, S.C.; Sharma, N.K.; Sharma, J.L. Systematic survey of some angiosperms of family Asteraceae from Kota district of Rajasthan, India-II. Int. J. Sci. Nat. 2014, 5, 183–185. [Google Scholar]
- Hussain, A.; Khan, M.N.; Iqbal, Z.; Sajid, M.S. An account of the botanical anthelmintics used in traditional veterinary practices in Sahiwal district of Punjab, Pakistan. J. Ethnopharmacol. 2008, 119, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.; Mossa, J.S.; El-Askary, H.I. Hirsutinolides from Vernonia cinerascens. Pharmazie 2000, 55, 144–145. [Google Scholar] [PubMed]
- Jakupovic, J.; Zdero, C.; Boeker, R.; Warning, U.; Bohlmann, F.; Jones, S.B. Vernocistifolide und andere Sesquiterpenlactone aus Vernonia und verwandten Arten. Liebigs Ann. Chem. 1987, 1987, 111–123. [Google Scholar]
- Ahmad, I.; Chaudhary, B.A.; Ashraf, M.; Uzair, M.; Hussain Janbaz, K. Vernonione, a new urease inhibitory carvotacetone derivative from Vernonia cinerascens. J. Chem. Soc. Pak. 2012, 34, 639–642. [Google Scholar]
- Rabe, T.; Mullholland, D.; Van Staden, J. Isolation and identification of antibacterial compounds from Vernonia colorata leaves. J. Ethnopharmacol. 2002, 80, 91–94. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Position | δC | δH (multi., J in Hz) | HMBC |
|---|---|---|---|
| 1 | 64.5, CH | 2.75, dd (9.8, 4.9) | 2, 3, 10 |
| 2 | 28.4, CH2 | α 2.24, ddt [tt] (13.9, 4.8) | 3, 10 |
| β 1.58, m | |||
| 3 | 23.37, CH2 | 2.51, td (13.5, 4.8) | 1, 2, 4, 5, 15 |
| 4 | 144.9, C | ||
| 5 | 149.3, CH | 6.35, dd (10.6, 1.0) | 3, 4, 7, 15 |
| 6 | 76.8, CH | 6.10, dd (10.6, 1.5) | 4, 7, 8, 11, 12 |
| 7 | 52.7, CH | 3.01, ddd [dq] (9.9, 1.7) | 5, 8, 9, 11, 12, 13 |
| 8 | 72.3, CH | 5.58, ddd (11.5, 9.9, 3.4) | 1′ |
| 9 | 47.2, CH2 | β 2.83, m | 1, 7, 8, 10, 14 |
| α 1.43, ddd (13.2, 11.5, 1.4) | |||
| 10 | 60.0, C | ||
| 11 | 135.8, C | ||
| 12 | 171.2, C | ||
| 13 | 131.4, CH2 | a 6.43, d (1.7) | 7, 8, 11, 12 |
| b 5.78, d (1.6) | |||
| 14 | 66.3, CH2 | 4.07, dd (11.0, 0.8) | 1, 9, 10 |
| 3.81, dd (11.0, 1.4) | |||
| 15 | 195.9, CH | 9.52, d (1.0) | 3, 4 |
| 1′ | 167.4, C | ||
| 2′ | 141.8, C | ||
| 3′ | 128.9, CH2 | a 6.21, dt [q] (0.9) | 1′, 2′, 4′ |
| b 5.89, dt [q] (1.3) | |||
| 4′ | 64.9, CH2 | 4.29, dd [t] (1.1) | 1′, 2′, 3′ |
| Compound | Tbr (µM) | Cytotoxicity (µM) | SI |
|---|---|---|---|
| 2 | 0.16 ± 0.04 | 5.6 ± 0.0 | 35 |
| 3 | 1.1 ± 0.3 | 4.7 ± 0.4 | 4.2 |
| 4 | 17 ± 0 | 13 ± 2 | 0.8 |
| 5 | 0.50 ± 0.01 | 6.9 ± 0.0 | 13 |
| 6 | 15 ± 0 | 49 ± 1 | 3.2 |
| 7 | 5.0 ± 0.0 | 22 ± 1 | 4.3 |
| 8 | 4.8 ± 1.1 | 128 ± 1 | 27 |
| PC b | 0.003 ± 0.001 | 0.007 ± 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Sesquiterpene Lactones from Vernonia cinerascens Sch. Bip. and Their in Vitro Antitrypanosomal Activity. Molecules 2018, 23, 248. https://doi.org/10.3390/molecules23020248
Kimani NM, Matasyoh JC, Kaiser M, Brun R, Schmidt TJ. Sesquiterpene Lactones from Vernonia cinerascens Sch. Bip. and Their in Vitro Antitrypanosomal Activity. Molecules. 2018; 23(2):248. https://doi.org/10.3390/molecules23020248
Chicago/Turabian StyleKimani, Njogu M., Josphat C. Matasyoh, Marcel Kaiser, Reto Brun, and Thomas J. Schmidt. 2018. "Sesquiterpene Lactones from Vernonia cinerascens Sch. Bip. and Their in Vitro Antitrypanosomal Activity" Molecules 23, no. 2: 248. https://doi.org/10.3390/molecules23020248
APA StyleKimani, N. M., Matasyoh, J. C., Kaiser, M., Brun, R., & Schmidt, T. J. (2018). Sesquiterpene Lactones from Vernonia cinerascens Sch. Bip. and Their in Vitro Antitrypanosomal Activity. Molecules, 23(2), 248. https://doi.org/10.3390/molecules23020248