Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes
Abstract
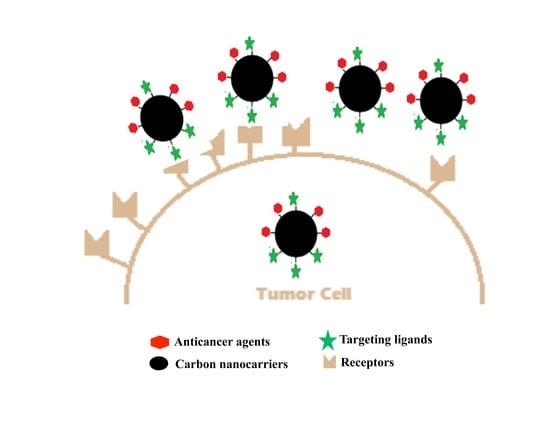
:1. Introduction
2. Drug Usage and Resistances in Cancer Cells
2.1. Doxorubicin-Loaded Carbon-Based Nanoparticles
2.2. Gemcitabine-Loaded Carbon-Based Nanoparticles
2.3. Other Drug Delivery Systems
2.4. Dual Drug Delivery and Synergistic Effects
3. Ligand-Receptor Mediated Delivery
3.1. Transferrin-Based Targeted Delivery
3.2. Folic Acid-Based Targeted Delivery
3.3. Hyaluronan-Based Targeted Delivery
4. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Han, X.; Li, S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon dots: Biomacromolecule interaction, bioimaging and nanomedicine. Coord. Chem. Rev. 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Margraf, J.T.; Strauss, V.; Guldi, D.M.; Clark, T. The electronic structure of amorphous carbon nanodots. J. Phys. Chem. B 2015, 119, 7258–7265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.H.; Du, C.; Zhuang, Z.H.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Sharma, S.K.; Peng, Z.L.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9. [Google Scholar] [CrossRef]
- Li, S.; Skromne, I.; Peng, Z.; Dallman, J.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. “Dark” carbon dots specifically “light-up” calcified zebrafish bones. J. Mater. Chem. B 2016, 4, 7398–7405. [Google Scholar] [CrossRef]
- Peng, Z.L.; Miyanji, E.H.; Zhou, Y.Q.; Pardo, J.; Hettiarachchi, S.D.; Li, S.H.; Blackwelder, P.L.; Skromne, I.; Leblanc, R.M. Carbon dots: Promising biomaterials for bone-specific imaging and drug delivery. Nanoscale 2017, 9, 17533–17543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Peng, Z.L.; Leblanc, R.M. Method to determine protein concentration in the protein nanoparticle conjugates aqueous solution using circular dichroism spectroscopy. Anal. Chem. 2015, 87, 6455–6459. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, S.; Han, X.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy. Anal. Chim. Acta 2016, 937, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, R. Carbon Quantum Dots; Springer International Publishing: Cham, Switzerland, 2017; p. 130. [Google Scholar]
- Han, X.; Li, S.H.; Peng, Z.L.; Al-Yuobi, A.R.O.; Bashammakh, A.S.O.; EI-Shahawi, M.S.; Leblanc, R.M. Interactions between carbon nanomaterials and biomolecules. J. Oleo Sci. 2016, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wang, L.Y.; Chusuei, C.C.; Suarez, V.M.; Blackwelder, P.L.; Micic, M.; Orbulescu, J.; Leblanc, R.M. Nontoxic carbon dots potently inhibit human insulin fibrillation. Chem. Mater. 2015, 27, 1764–1771. [Google Scholar] [CrossRef]
- Wang, B.B.; Wang, S.J.; Wang, Y.F.; Lv, Y.; Wu, H.; Ma, X.J.; Tan, M.Q. Highly fluorescent carbon dots for visible sensing of doxorubicin release based on efficient nanosurface energy transfer. Biotechnol. Lett. 2016, 38, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Hu, A.G. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Duan, W.X.; Song, W.; Liu, J.J.; Ren, C.L.; Wu, J.; Liu, D.; Chen, H.L. Red emission B, N, S-co-doped carbon dots for colorimetric and fluorescent dual mode detection of Fe3+ ions in complex biological fluids and living cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.X.; Zhu, J.Y.; Zhai, Y.; Wang, H.; Bai, X.; Dong, B.; Wang, H.Y.; Song, H.W. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042–13051. [Google Scholar] [CrossRef] [PubMed]
- De Moor, J.S.; Mariotto, A.B.; Parry, C.; Alfano, C.M.; Padgett, L.; Kent, E.E.; Forsythe, L.; Scoppa, S.; Hachey, M.; Rowland, J.H. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol. Biomark. Prev. 2013, 22, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bjornmalm, M.; Caruso, F. Particle carriers for combating multidrug-resistant cancer. ACS Nano 2013, 7, 9512–9517. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging-US 2009, 1, 988–1007. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.R.; Bae, Y.; Iwata, C.; Morishita, Y.; Yashiro, M.; Oka, M.; Fujii, T.; Komuro, A.; Kiyono, K.; Kaminishi, M.; et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 3460–3465. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Ou, C.M.; Huang, C.C.; Wu, W.C.; Chen, Y.P.; Lin, T.E.; Ho, L.C.; Wang, C.W.; Shih, C.C.; Zhou, H.C.; et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J. Mater. Chem. B 2014, 2, 4564–4571. [Google Scholar] [CrossRef]
- Hsu, P.C.; Chen, P.C.; Ou, C.M.; Chang, H.Y.; Chang, H.T. Extremely high inhibition activity of photoluminescent carbon nanodots toward cancer cells. J. Mater. Chem. B 2013, 1, 1774–1781. [Google Scholar] [CrossRef]
- Yu, B.; Tai, H.C.; Xue, W.M.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, F. Combating P-glycoprotein-mediated multidrug resistance using therapeutic nanoparticles. Curr. Pharm. Des. 2013, 19, 6655–6666. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.M.; Blackwelder, P.L.; Leblanc, R.M. Crossing the blood-brain barrier with transferrin conjugated carbon dots: A zebrafish model study. Colloids Surf. B Biointerfaces 2016, 145, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Trang, P.; Wiggins, J.F.; Daige, C.L.; Cho, C.; Omotola, M.; Brown, D.; Weidhaas, J.B.; Bader, A.G.; Slack, F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011, 19, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Yang, D.; Yang, F.; Hu, J.H.; Long, J.; Wang, C.C.; Fu, D.L.; Ni, Q.X. Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehicles. Chem. Commun. 2009, 2009, 4447–4449. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Meng, L.J.; Lu, Q.H. Folate-conjugated peg on single walled carbon nanotubes for targeting delivery of doxorubicin to cancer cells. Macromol. Biosci. 2013, 13, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Ansari, S.; Milone, C.; Salamo, M.; Galvagno, S.; Cirmi, S.; Navarra, M. Tunable doxorubicin release from polymer-gated multiwalled carbon nanotubes. Int. J. Pharm. 2016, 515, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Meng, L.J.; Lu, Q.H.; Fei, Z.F.; Dyson, P.J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.L.; Liu, H.Q.; Tan, H.R.; Liu, Y. Delivery of paclitaxel by physically loading onto poly(ethyleneglycol) (PEG)-graftcarbon nanotubes for potent cancer therapeutics. Nanotechnology 2010, 21, 065101. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, V.S.; Donnenberg, A.D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R. The Biology of Cancer; Garland Science: New York, NY, USA, 2013. [Google Scholar]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, X.Y.; Ng, V.W.L.; Gao, S.J.; Tong, Y.W.; Hedrick, J.L.; Yang, Y.Y. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials 2014, 35, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. PEGylated oxidized alginate-Dox prodrug conjugate nanoparticles cross-linked with fluorescent carbon dots for tumor theranostics. ACS Biomater. Sci. Eng. 2016, 2, 1641–1648. [Google Scholar] [CrossRef]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; De Angulo, G.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mehra, N.K.; Jain, V.; Jain, N.K. Gemcitabine-loaded smart carbon nanotubes for effective targeting to cancer cells. J. Drug Target. 2013, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Sharma, G.; Sonali; Singh, S.; Patne, S.C.U.; Pandey, B.L.; Koch, B.; Muthu, M.S. Effects of transferrin conjugated multi-walled carbon nanotubes in lung cancer delivery. Mater. Sci. Eng. C 2016, 67, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.R.; Wang, J.; Jiang, W.H.; Jiang, X.W.; Bai, Z.S.; He, Y.P.; Jiang, J.Q.; Wang, D.K.; Yang, L. Doxorubicin conjugated functionalizable carbon dots for nucleus targeted delivery and enhanced therapeutic efficacy. Nanoscale 2016, 8, 6801–6809. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zheng, M.; Xie, Z.; Jing, X. Supramolecular hybrids of carbon dots with doxorubicin: Synthesis, stability and cellular trafficking. Mater. Chem. Front. 2017, 1, 354–360. [Google Scholar] [CrossRef]
- Mewada, A.; Pandey, S.; Thakur, M.; Jadhav, D.; Sharon, M. Swarming carbon dots for folic acid mediated delivery of doxorubicin and biological imaging. J. Mater. Chem. B 2014, 2, 698–705. [Google Scholar] [CrossRef]
- Song, Y.C.; Shi, W.; Chen, W.; Li, X.H.; Ma, H.M. Fluorescent carbon nanodots conjugated with folic acid for distinguishing folate-receptor-positive cancer cells from normal cells. J. Mater. Chem. 2012, 22, 12568–12573. [Google Scholar] [CrossRef]
- Zhao, X.W.; Zhang, J.L.; Shi, L.H.; Xian, M.; Dong, C.; Shuang, S.M. Folic acid-conjugated carbon dots as green fluorescent probes based on cellular targeting imaging for recognizing cancer cells. RSC Adv. 2017, 7, 42159–42167. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhang, J.; Liu, Y.H.; Luo, T.Y.; He, X.; Yu, X.Q. Hyaluronic acid-based carbon dots for efficient gene delivery and cell imaging. RSC Adv. 2017, 7, 15613–15624. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Shah, J.S.; Misra, R.D.K. A new family of folate-decorated and carbon nanotube-mediated drug delivery system: Synthesis and drug delivery response. Adv. Drug Deliv. Rev. 2011, 63, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Datir, S.R.; Das, M.; Singh, R.P.; Jain, S. Hyaluronate tethered, “smart” multiwalled carbon nanotubes for tumor-targeted delivery of doxorubicin. Bioconjugate Chem. 2012, 23, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, C.; Yang, D.; Jiang, Y.J.; Li, J.; Di, Y.; Hu, J.H.; Wang, C.C.; Ni, Q.X.; Fu, D.L. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer 2011, 47, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wang, M.L.; Miao, F.; Ji, Y.X.; Tian, Z.; Shen, H.B.; Jia, N.Q. Water-dispersible multiwalled carbon nanotube/iron oxide hybrids as contrast agents for cellular magnetic resonance imaging. Carbon 2012, 50, 2162–2170. [Google Scholar] [CrossRef]
- Lamprecht, C.; Gierlinger, N.; Heister, E.; Unterauer, B.; Plochberger, B.; Brameshuber, M.; Hinterdorfer, P.; Hild, S.; Ebner, A. Mapping the intracellular distribution of carbon nanotubes after targeted delivery to carcinoma cells using confocal Raman imaging as a label-free technique. J. Phys. Condens. Matter 2012, 24, 164206. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H.J. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.J.; Zhang, Y.G.; Sun, L.; Liu, Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials 2014, 35, 9208–9223. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.L.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.E.; Randolph, J.K.; Gewirtz, D.A. Antagonism between Tamoxifen and Doxorubicin in the Mcf-7 Human Breast-Tumor Cell-Line. Biochem. Pharmacol. 1994, 47, 1449–1452. [Google Scholar] [CrossRef]
- Kim, D.; Choi, Y.; Shin, E.; Jung, Y.K.; Kim, B.S. Sweet nanodot for biomedical imaging: Carbon dot derived from xylitol. RSC Adv. 2014, 4, 23210–23213. [Google Scholar] [CrossRef]
- Motlagh, N.S.H.; Parvin, P.; Ghasemi, F.; Atyabi, F. Fluorescence properties of several chemotherapy drugs: Doxorubicin, paclitaxel and bleomycin. Biomed. Opt. Express 2016, 7, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Du, F.Y.; Liu, P.C.; Chen, Z.J.; Shen, J.C. DNA-carbon dots function as fluorescent vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Park, S.Y.; Park, E.S.; Son, B.; Lee, S.C.; Lee, J.W.; Lee, Y.C.; Kang, K.S.; Kim, M.I.; Park, H.G.; et al. Photoluminescent carbon nanotags from harmful cyanobacteria for drug delivery and imaging in cancer cells. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.F.; Meydanli, M.M.; Tulunay, G. Gemcitabine plus carboplatin in platinum-sensitive recurrent ovarian carcinoma. Expert Rev. Anticancer Ther. 2006, 6, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.F.S.A.; Fernandes, P.A.; Ramos, M.L. Understanding ribonucleotide reductase inactivation by gemcitabine. Chem. Eur. J. 2007, 13, 8507–8515. [Google Scholar] [CrossRef] [PubMed]
- Winer, E.; Gralow, J.; Diller, L.; Karlan, B.; Loehrer, P.; Pierce, L.; Demetri, G.; Ganz, P.; Kramer, B.; Kris, M.; et al. Clinical cancer advances 2008: Major research advances in cancer treatment, prevention, and screening-a report from the american society of clinical oncology. J. Clin. Oncol. 2009, 27, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Arsawang, U.; Saengsawang, O.; Rungrotmongkol, T.; Sornmee, P.; Wittayanarakul, K.; Remsungnen, T.; Hannongbua, S. How do carbon nanotubes serve as carriers for gemcitabine transport in a drug delivery system? J. Mol. Graph. Model. 2011, 29, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Mariano, L.; Salmaso, S.; Caliceti, P.; Gaetano, G. Folate-mediated targeting of polymeric conjugates of gemcitabine. Int. J. Pharm. 2006, 307, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Sharker, S.M.; Kim, S.M.; Kim, S.H.; In, I.; Lee, H.; Park, S.Y. Target delivery of beta-cyclodextrin/paclitaxel complexed fluorescent carbon nanoparticles: Externally NIR light and internally pH sensitive-mediated release of paclitaxel with bio-imaging. J. Mater. Chem. B 2015, 3, 5833–5841. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.Z.; Chen, X.Y.; Dai, H.J. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Ajima, K.; Murakami, T.; Mizoguchi, Y.; Tsuchida, K.; Ichihashi, T.; Iijima, S.; Yudasaka, M. Enhancement of in vivo anticancer effects of cisplatin by incorporation inside single-wall carbon nanohorns. ACS Nano 2008, 2, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Ponde, N. Cancer drugs, survival and ethics: A critical look from the inside. ESMO Open 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Scarano, W.; de Souza, P.; Stenzel, M.H. Dual-drug delivery of curcumin and platinum drugs in polymeric micelles enhances the synergistic effects: A double act for the treatment of multidrug-resistant cancer. Biomater. Sci. 2015, 3, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.P.; Meziani, M.J.; Sun, Y.P.; Cheng, S.H. Poly(ethylene glycol)-conjugated multi-walled carbon nanotubes as an efficient drug carrier for overcoming multidrug resistance. Toxicol. Appl. Pharmacol. 2011, 250, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Zou, X.Y.; Yang, L.L.; Lin, S.; Fan, J.; Yang, B.; Sun, X.Y.; Wan, Q.; Chen, Y.; Fu, S.Z. Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids Surf. B Biointerfaces 2014, 122, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cai, C.H.; Lin, J.P.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Heister, E.; Neves, V.; Lamprecht, C.; Silva, S.R.P.; Coley, H.M.; McFadden, J. Drug loading, dispersion stability, and therapeutic efficacy in targeted drug delivery with carbon nanotubes. Carbon 2012, 50, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.M.; Xia, J.G.; Zhao, Q.H.; Liu, L.W.; Zhang, Z.J. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; You, G.X.; Xu, Y.; Wang, C.; Wang, P.F.; Miao, L.Z.; Dai, S.S.; Lv, B.W.; Yang, Y.Y. Antioxidant enzyme activities as biomarkers of fluvial biofilm to ZnO NPs ecotoxicity and the Integrated Biomarker Responses (IBR) assessment. Ecotoxicol. Environ. Saf. 2016, 133, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Young, S.W.S.; Stenzel, M.; Yang, J.L. Nanoparticle-siRNA: A potential cancer therapy? Crit. Rev. Oncol. Hemat. 2016, 98, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.H.; Deng, C.; Klok, H.A.; Zhong, Z.Y. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Lu, Y.J.; Lin, K.J.; Hsu, S.C.; Huang, C.Y.; She, S.H.; Liu, H.L.; Lin, C.W.; Xiao, M.C.; Wey, S.P.; et al. EGRF conjugated PEGylated nanographene oxide for targeted chemotherapy and photothermal therapy. Biomaterials 2013, 34, 7204–7214. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Elliott, R.L.; Head, J.F. Cancer: Tumor iron metabolism, mitochondrial dysfunction and tumor immunosuppression; “A tight partnership—was Warburg correct?”. J. Cancer Ther. 2012, 3, 278. [Google Scholar] [CrossRef]
- Warner, F.W.; Stjernholm, R.; Cohn, I. Electron paramagnetic resonance investigation of high-spin iron (III) in cancer. Med. Phys. 1978, 5, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Li, H.Y.; Sun, H.Z.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.; Karagiannis, T.C. Transferrin receptor-mediated endocytosis: A useful target for cancer therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Fritzer, M.; Barabas, K.; Szuts, V.; Berczi, A.; Szekeres, T.; Faulk, W.P.; Goldenberg, H. Cytotoxicity of a transferrin-adriamycin conjugate to anthracycline-resistant cells. Int. J. Cancer 1992, 52, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Fritzer, M.; Szekeres, T.; Szuts, V.; Jarayam, H.N.; Goldenberg, H. Cytotoxic effects of a doxorubicin-transferrin conjugate in multidrug-resistant KB cells. Biochem. Pharmacol. 1996, 51, 489–493. [Google Scholar] [CrossRef]
- Lubgan, D.; Jozwiak, Z.; Grabenbauer, G.G.; Distel, L.V.R. Doxorubicin-transferrin conjugate selectively overcomes multidrug resistance in leukaemia cells. Cell. Mol. Biol. Lett. 2009, 14, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Loo, R.R.O.; Loo, J.A. Mass spectrometric characterization of transferrins and their fragments derived by reduction of disulfide bonds. J. Am. Soc. Mass. Spectrom. 2003, 14, 635–647. [Google Scholar] [CrossRef]
- Wood, J.C. Magnetic resonance imaging measurement of iron overload. Curr. Opin. Hematol. 2007, 14, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Okazaki, Y.; Shi, L.; Kohda, H.; Tanaka, M.; Taki, K.; Nishioka, T.; Hirayama, T.; Nagasawa, H.; Yamashita, Y.; et al. Role of hemoglobin and transferrin in multi-wall carbon nanotube-induced mesothelial injury and carcinogenesis. Cancer Sci. 2016, 107, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.; Kim, J.H.; Park, K.; Kim, K.; Kwon, I.C. Polymeric nanomedicine for cancer therapy. Prog. Polym. Sci. 2008, 33, 113–137. [Google Scholar] [CrossRef]
- Leamon, C.P.; Low, P.S. Delivery of macromolecules into living cells—A method that exploits folate receptor endocytosis. Proc. Natl. Acad. Sci. USA 1991, 88, 5572–5576. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for Imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Garinchesa, P.; Campbell, I.; Saigo, P.E.; Lewis, J.L.; Old, L.J.; Rettig, W.J. Trophoblast and ovarian-cancer antigen-Lk26—Sensitivity and specificity in immunopathology and molecular-identification as a folate-binding protein. Am. J. Pathol. 1993, 142, 557–567. [Google Scholar]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Quere, R.; Andradottir, S.; Brun, A.C.M.; Zubarev, R.A.; Karlsson, G.; Olsson, K.; Magnusson, M.; Cammenga, J.; Karlsson, S. High levels of the adhesion molecule CD44 on leukemic cells generate acute myeloid leukemia relapse after withdrawal of the initial transforming event. Leukemia 2011, 25, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Bourguignon, L.Y.W. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011, 178, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Earle, C.; Wong, G.; Bourguignon, L.Y.W. Role of hyaluronan synthase 2 to promote CD44-dependent oral cavity squamous cell carcinoma progression. Head Neck 2013, 35, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Adamo, H.; Hammarsten, P.; Granfors, T.; Stattin, P.; Egevad, L.; Laurent, A.E.; Wikstrom, P.; Bergh, A. Prostate cancer increases hyaluronan in surrounding nonmalignant stroma, and this response is associated with tumor growth and an unfavorable outcome. Am. J. Pathol. 2011, 179, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, P.G.; Ilina, O.; Friedl, P. Interstitial guidance of cancer invasion. J. Pathol. 2012, 226, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Basakran, N.S. CD44 as a potential diagnostic tumor marker. Saudi Med. J. 2015, 36, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zwick, E.; Bange, J.; Ullrich, A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr.-Relat. Cancer 2001, 8, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Yu, X.Y.; Wang, L.; Liu, Y.; Gao, J.; Zhang, J.; Ma, R.; Liu, R.Y.; Zhang, Z.Z. PEGylated fullerene/iron oxide nanocomposites for photodynamic therapy, targeted drug delivery and MR imaging. Biomaterials 2013, 34, 9666–9677. [Google Scholar] [CrossRef] [PubMed]
- Abdullah-Al-Nahain; Lee, J.E.; In, I.; Lee, H.; Lee, K.D.; Jeong, J.H.; Park, S.Y. Target delivery and cell imaging using hyaluronic acid-functionalized graphene quantum dots. Mol. Pharm. 2013, 10, 3736–3744. [Google Scholar]
- Fischer, J.; Ganellin, C.R. Analogue-Based Drug Discovery II; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]








| Carbon Nanoparticles | Drug Loaded | Ligand Attached | Cell Targeted | Characterization Method | Drug Loading | Reference |
|---|---|---|---|---|---|---|
| C-dots | Dox | Nuclear localization signal peptide | A549 | AFM, TEM, XPS, UV-Vis, Fluorescence, Confocal, Flow cytometry, FTIR, NMR | - | [44] |
| C-dots | Dox | - | HeLa | UV-Vis, Fluorescence, XPS, TEM, FTIR, Zeta | - | [14] |
| C-dots | Dox | - | HeLa | UV-Vis; Zeta; DLS; PL; TEM | 260% | [45] |
| C-dots | Dox | Transferrin | CHLA-266, SJGBM2 | Fluorescence, UV-Vis, MALDI-TOF | - | [41] |
| C-dots | Dox | Folic acid | HeLa | FTIR, UV-Vis, Zeta | 85.6% | [46] |
| C-dots | - | Folic acid | HeLa, NIH-3T3, MCF-7 | Fluorescence, TEM, UV-Vis | - | [47] |
| C-dots | - | Folic acid | HepG-2 | UV-Vis, Fluorescence, FTIR, TEM, XPS | - | [48] |
| C-dots | Gene | Hyaluronan | HeLa | FTIR, NMR, UV-Vis, Fluorescence, TEM | - | [49] |
| CNTs | Dox | - | SH-SY5Y, HT-29, HepG-2 | FTIR, TEM | [33] | |
| CNTs | Dox | Folic acid | HeLa, 3T3 | UV-Vis, IR, TEM, Zeta | 149.3% | [32] |
| CNTs | Dox | Folic acid | HeLa | UV-Vis, TEM | - | [34] |
| CNTs | Dox | Folic acid | - | UV-Vis, Fluorescence, FTIR, SEM | 91% | [50] |
| CNTs | Dox | Hyaluronan | - | SEM, TEM, Zeta, FTIR | - | [51] |
| CNTs | Gem | Folic acid | Breast cancer cells | Electron microscopy, FT-IR, X-ray diffraction | - | [42] |
| CNTs | Gem | - | FT-IR, NMR | - | [52] | |
| CNTs | Docetaxel | Transferrin | A549 | AFM, FTIR, TEM, Zeta | - | [43] |
| CNTs | - | Folic acid | Hela | UV-Vis, TEM, Zeta | - | [53] |
| CNTs | - | Folic acid | T24 | AFM, TEM, Raman spectra | - | [54] |
| CNTs | - | Folic acid | HeLa | UV-Vis, AFM, Confocal, Fluorescence, SEM | - | [55] |
| CNTs | - | Hyaluronan | Gastric cancer stem cells | UV-Vis, Confocal, Flow Cytometry | - | [56] |
| Carbon Source | Drug Loaded | Ligand Attached | Targeted Cell | Reference |
|---|---|---|---|---|
| C-dots | Dox | Transferrin | CHLA-266, SJGBM2 | [41] |
| C-dots | Dox | Folic acid | HeLa | [46] |
| C-dots | - | Folic acid | HeLa, NIH-3T3, MCF-7 | [47] |
| CNTs | Docetaxel | Transferrin | A549 | [43] |
| CNTs | Gem | Folic acid | Breast cancer cells | [42] |
| CNTs | - | Folic acid | Hela | [53] |
| CNTs | - | Folic acid | T24 | [54] |
| CNTs | Dox | Folic acid | HeLa | [34] |
| CNTs | - | Folic acid | HeLa | [55] |
| CNTs | - | Hyaluronan | Gastric cancer stem cells | [56] |
| CNTs | Dox | Hyaluronan | - | [51] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules 2018, 23, 378. https://doi.org/10.3390/molecules23020378
Pardo J, Peng Z, Leblanc RM. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules. 2018; 23(2):378. https://doi.org/10.3390/molecules23020378
Chicago/Turabian StylePardo, Joel, Zhili Peng, and Roger M. Leblanc. 2018. "Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes" Molecules 23, no. 2: 378. https://doi.org/10.3390/molecules23020378
APA StylePardo, J., Peng, Z., & Leblanc, R. M. (2018). Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules, 23(2), 378. https://doi.org/10.3390/molecules23020378