Synthesis, Characterization, Crystal Structure, and DFT Study of a New Square Planar Cu(II) Complex Containing Bulky Adamantane Ligand
Abstract
:1. Introduction
2. Results and Discussion
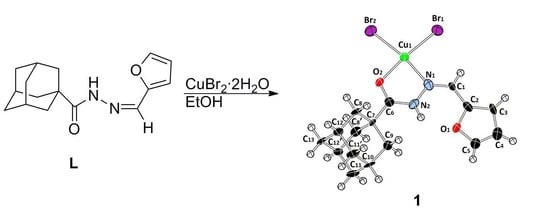
2.1. Preparation and Characterization of the Copper(II) Complex
2.2. Description of the Crystal Structure
2.3. DFT Calculations
3. Materials and Methods
3.1. Synthesis of the Copper(II) Complex 1
3.2. X-ray Difraction Study
3.3. Computational Details
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rollas, S.; Gulerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2, 5-disubstituted-1, 3, 4-oxadiazolines. Il Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef]
- Bedia, K.; Elçin, O.; Seda, U.; Fatma, K.; Nathaly, S.; Sevim, R.; Dimoglo, A. Synthesis and characterization of novel hydrazide–hydrazones and the study of their structure–antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. Hydrazones of 1, 2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002, 37, 553–564. [Google Scholar] [CrossRef]
- Ersan, S.; Nacak, S.; Berkem, R.; Ozden, T. Synthesis and antimicrobial activities of 2-[(alpha-methylbenzylidene)-hydrazino] benzoxazoles. Arzneim.-Forsch. 1997, 47, 963–965. [Google Scholar]
- Yildir, I.; Perçiner, H.; Sahin, M.F.; Abbasoglu, U. Hydrazones of [(2-Benzothiazolylthio) acetyl] hydrazine: Synthesis and Antimicrobial Activity. Arch. Pharm. 1995, 328, 547–549. [Google Scholar] [CrossRef]
- Cesur, Z.; Büyüktimkin, S.; Büyüktimkin, N.; Derbentli, S. Synthesis and Antimicrobial Evaluation of Some Arylhydrazones of 4-[(2-Methylimidazo [1, 2-a] pyridine-3-yl) azo] benzoic Acid Hydrazide. Arch. Pharm. 1990, 323, 141–144. [Google Scholar] [CrossRef]
- Vittorio, F.; Ronsisvalle, G.; Marrazzo, A.; Blandini, G. Synthesis and antimicrobial evaluation of 4-phenyl-3-isoquinolinoyl-hydrazones. Il Farmaco 1995, 50, 265–272. [Google Scholar] [PubMed]
- Rasras, A.; Al-Tel, T.; Al-Aboudi, A.; Al-Qawasmeh, R. Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur. J. Med. Chem. 2010, 45, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Incerti, M.; Doytchinova, I.A.; La Colla, P.; Busonera, B.; Loddo, R. Synthesis and antiproliferative activity of benzo [d] isothiazole hydrazones. Eur. J. Med. Chem. 2006, 41, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, G.A.; El-Asmy, A.A. Synthesis, spectroscopy and thermal analysis of copper (II) hydrazone complexes. J. Coord. Chem. 2009, 62, 337–345. [Google Scholar] [CrossRef]
- Dawkins, A.T.; Gallager, L.R.; Togo, Y.; Hornick, R.B.; Harris, B.A. Studies on induced influenza in man: II. Double-blind study designed to assess the prophylactic efficacy of an analogue of amantadine hydrochloride. J. Am. Med. Assoc. 1968, 203, 1095–1099. [Google Scholar] [CrossRef]
- Çalıs, Ü.; Yarıma, M.; Köksal, M.; Özalp, M. Synthesis and antimicrobial activity evaluation of some new adamantane derivatives. Arzneim.-Forsch. 2002, 52, 778–781. [Google Scholar] [CrossRef]
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1, 3, 4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou-Vyza, E.; Avramidis, N.; Kourounakis, A.; Hadjipetrou, L. Anti-inflammatory properties of new adamantane derivatives. Design, synthesis, and biological evaluation. Arch. Pharm. Pharm. Med. Chem. 1998, 331, 72–78. [Google Scholar] [CrossRef]
- Olson, S.; Aster, S.D.; Brown, K.; Carbin, L.; Graham, D.W.; Hermanowski-Vosatka, A.; LeGrand, C.B.; Mundt, S.S.; Robbins, M.A.; Schaeffer, J.M.; et al. Adamantyl triazoles as selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1. Bioorg. Med. Chem. Lett. 2005, 15, 4359–4362. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.; Dinodiaa, M.; Kumar, A. Synthesis, anti-inflammatory and analgesic activity evaluation of some amidine and hydrazone derivatives. Bioorg. Med. Chem. 2006, 14, 4657–4663. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez, J.M.; Acevedo-Arauz, E.; Cetina-Rosado, R.; Macías-Ruvalcaba, N.; Toscano, R.A. Electrochemical studies of copper (II) complexes derived from bulky Schiff bases. The crystal structure of bis [N-(1-adamantyl)-salicylaldiminato] copper (II). Trans. Met. Chem. 1999, 24, 18–24. [Google Scholar] [CrossRef]
- Đorđević, M.; Jeremić, D.; Anđelković, K.; Pavlović, M.; Divjaković, V.; Ristović, M.; Brčeski, I. Cobalt (II) and cadmium (II) compounds with adamantane-1-sulfonic acid. J. Serb. Chem. Soc. 2012, 77, 1391–1399. [Google Scholar] [CrossRef]
- Franco, J.; Olmstead, M.; Hammons, J. Bis[1-(1-adamantyliminomethyl)-2-naphtholato-κ2N, O] cobalt (II). Acta Crystallogr. Sect. E 2008, 64, m1223. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Karlin, K.D. Model complexes for copper-containig enzymes. In Concepts and Models in Bioinorganic Chemistry; Kraatz, H.-B., Metzler-Nolte, N., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 363–396. ISBN 9783527313051. [Google Scholar]
- Lippard, S.J.; Berg, J.M. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994; pp. 1–6. ISBN 0935702725. [Google Scholar]
- Da Silva, J.F.; Williams, R.J.P. The Biological Chemistry of the Elements: the Inorganic Chemistry of Life, 2nd ed.; Clarendon: Oxford, UK, 2001; pp. 7–26. ISBN 9780198508489. [Google Scholar]
- Teyssot, M.-L.; Jarrousse, A.-S.; Chevry, A.; De Haze, A.; Beaudoin, C.; Manin, M.; Nolan, S.P.; Diez-Gonzalez, S.; Morel, L.; Gautier, A. Toxicity of Copper (I)–NHC Complexes Against Human Tumor Cells: Induction of Cell Cycle Arrest, Apoptosis, and DNA Cleavage. Chem. Eur. J. 2009, 15, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGivern, T.J.P.; Afsharpour, S.; Marmion, C.J. Copper complexes as artificial DNA metallonucleases: From Sigman’s reagent to next generation anti-cancer agent? Inorg. Chim. Acta 2017, 472, 12–39. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Akitsu, T. A Metal Complex Incorporating Some Drug Molecules as Ligands. EC Pharmacol. Toxicol. 2017, ECO.01, 16–18. [Google Scholar]
- Al-Qawasmeh, R.A.; Salameh, B.; Alrazim, R.; Aldamen, M.; Voelter, W. Microwave Assisted Synthesis of New Adamantyltriazine Derivatives. Lett. Org. Chem. 2014, 11, 513–518. [Google Scholar] [CrossRef]
- Al-Aboudi, A.; Al-Qawasmeh, R.A.; Shahwan, A.; Mahmood, U.; Khalid, A.; Ul-Haq, Z. In-silico identification of the binding mode of synthesized adamantyl derivatives inside cholinesterase enzymes. Acta Pharmacol. Sin. 2015, 36, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Q.; Li, Y.; Zhang, D.-Y.; Nie, Y.; Li, Z.-J.; Gu, W.; Liu, X.; Tian, J.-L.; Yan, S.-P. Copper complexes based on chiral Schiff-base ligands: DNA/BSA binding ability, DNA cleavage activity, cytotoxicity and mechanism of apoptosis. Eur. J. Med. Chem. 2016, 114, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Koval’chukovaa, O.V.; Zavodnikb, V.E.; Shestakovc, A.F.; Strashnovaa, S.B.; Zaitseva, B.E. Experimental and Theoretical Investigation of the Structure and Spectral Characteristics of Bis(4–aza–9–fluorenone)dibromocopper(II). Russ. J. Inorg. Chem. 2010, 55, 195–200. [Google Scholar] [CrossRef]
- Vela, S.; Deumal, M.; Turnbull, M.M.; Novoa, J.J. A theoretical analysis of the magnetic properties of the low-dimensional copper(II)X2(2-X-3-methylpyridine)2(X = Cl and Br) complexes. Theor. Chem. Acc. 2013, 132, 1331. [Google Scholar] [CrossRef]
- Posada, N.B.; Guimarães, M.A.; Padilha, D.S.; Resende, J.A.; Faria, R.B.; Lanznaster, M.; Amado, R.S.; Scarpellini, M. Influence of the secondary coordination sphere on the physical properties of mononuclear copper (II) complexes and their catalytic activity on the oxidation of 3, 5-di-tert-butylcatechol. Polyhedron 2018, 141, 30–36. [Google Scholar] [CrossRef]
- Piri, Z.; Moradi-Shoeili, Z.; Assoud, A. New copper (II) complex with bioactive 2–acetylpyridine-4N-p-chlorophenylthiosemicarbazone ligand: Synthesis, X-ray structure, and evaluation of antioxidant and antibacterial activity. Inorg. Chem. Commun. 2017, 84, 122–126. [Google Scholar] [CrossRef]
- Thomas, F.; Kochem, A.; Molloy, J.K.; Gellon, G.; Leconte, N.; Philouze, C.; Jarjayes, O.; Berthiol, F. A structurally characterized Cu (III) complex supported by a bis (anilido) ligand and its oxidative catalytic activity. Chem. Eur. J. 2017, 23, 13929–13940. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Parvesh, P.; Sharma, C.; Aneja, K. Metal-Based Biologically Active Compounds: Synthesis, Spectral, and Antimicrobial Studies of Cobalt, Nickel, Copper, and Zinc Complexes of Triazole-Derived Schiff Bases. Bioinorg. Chem. Appl. 2011, 2011, 901716–901726. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis Software System; Version 1.171; Oxford Diffraction Ltd.: Oxford, England, 2002.
- SHELXL; Version 97; Program for X-ray Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997.
- Images generated using CrystalMaker(R), version 9; A crystal and molecular structures program for Mac and Windows (www.crystalmaker.com); CrystalMaker Software Ltd.: Oxford, UK, 2015.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 03; Revision 05; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
Sample Availability: Samples of the compound 1 is available from the authors. |







| Crystal System | Monoclinic |
|---|---|
| T/K | 293(2) |
| Space group | P21/m |
| a/Å | 10.8030(8) |
| b/Å | 6.6115(8) |
| c/Å | 12.1264(12) |
| β/° | 101.124(8) |
| Volume/Å3 | 849.9(3) |
| Z | 2 |
| ρcalc g/cm3 | 1.937 |
| μ/mm−1 | 5.997 |
| F(000) | 490 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 6.848–51.348 |
| Index ranges | −13 ≤ h ≤ 13, −5 ≤ k ≤ 8, −13 ≤ l ≤ 14 |
| Reflections collected | 3640 |
| Independent reflections | 1754 [Rint = 0.0842, Rsigma = 0.1264] |
| Data/restraints/parameters | 1754/0/130 |
| Goodness-of-fit on F2 | 1.017 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0751, wR2 = 0.1581 |
| Final R indexes [all data] | R1 = 0.1214, wR2 = 0.1902 |
| Largest diff. peak/hole / e Å−3 | 1.12/−1.07 |
| Exp. | Theo. | Exp. | Theo. | ||||
| dimer | monomer | dimer | monomer | ||||
| Cu1-Br1 | 2.376(2) | 2.379 | 2.287 | C7-C6 | 1.505(15) | 1.521 | 1.935 |
| Cu1-Br2 | 2.368(2) | 2.323 | 2.298 | C7C8 | 1.519(10) | 1.556 | 1.556 |
| Cu1-O2 | 1.987(8) | 2.094 | 2.036 | C7-C9 | 1.574(13) | 1.548 | 1.556 |
| Cu1-N1 | 2.067(10) | 2.094 | 2.061 | C4-C5 | 1.307(17) | 1.364 | 1.364 |
| O2-C6 | 1.268(13) | 1.241 | 1.243 | C4-C3 | 1.460(17) | 1.424 | 1.423 |
| O1-C2 | 1.385(14) | 1.382 | 1.382 | C2-C3 | 1.351(17) | 1.373 | 1.375 |
| O1-C5 | 1.364(14) | 1.362 | 1.361 | C8-C12 | 1.521(11) | 1.545 | 1.545 |
| N2-N1 | 1.381(13) | 1.376 | 1.372 | C10-C9 | 1.515(16) | 1.541 | 1.541 |
| N2-C6 | 1.320(15) | 1.357 | 1.364 | C10-C11 | 1.518(11) | 1.541 | 1.543 |
| N1-C1 | 1.250(14) | 1.295 | 1.294 | C13-C12 | 1.526(10) | 1.542 | 1.543 |
| C1-C2 | 1.457(16) | 1.433 | 1.432 | C12-C11 | 1.510(9) | 1.542 | 1.542 |
| Cu1···Cu1 | 3.985 | 3.258 | - | N2···O1 | 2.665 | 2.755 | 2.768 |
| Cu1···Br1 | 3.385 | 2.623 | - | N1-Cu1-Br1 | 93.7(3) | 93.39 | 96.55 |
| Br2-Cu1-Br1 | 97.03(7) | 93.50 | 109.09 | N1-Cu1-Br2 | 169.3(3) | 156.42 | 138.04 |
| O2-Cu1-Br1 | 173.9(2) | 163.45 | 147.02 | Br1···N1N2C6O2 | 0.000 | −0.012 | −0.538 |
| O2-Cu1-Br2 | 89.0(2) | 90.45 | 95.43 | Br2···N1N2C6O2 | 0.000 | −0.028 | 2.109 |
| O2-Cu1-N1 | 80.3(4) | 77.04 | 77.93 | Cu1···N1N2C6O2 | 0.000 | 0.277 | 0.355 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanfar, M.A.; Jaber, A.M.; AlDamen, M.A.; Al-Qawasmeh, R.A. Synthesis, Characterization, Crystal Structure, and DFT Study of a New Square Planar Cu(II) Complex Containing Bulky Adamantane Ligand. Molecules 2018, 23, 701. https://doi.org/10.3390/molecules23030701
Khanfar MA, Jaber AM, AlDamen MA, Al-Qawasmeh RA. Synthesis, Characterization, Crystal Structure, and DFT Study of a New Square Planar Cu(II) Complex Containing Bulky Adamantane Ligand. Molecules. 2018; 23(3):701. https://doi.org/10.3390/molecules23030701
Chicago/Turabian StyleKhanfar, Monther A., Areej M. Jaber, Murad A. AlDamen, and Raed A. Al-Qawasmeh. 2018. "Synthesis, Characterization, Crystal Structure, and DFT Study of a New Square Planar Cu(II) Complex Containing Bulky Adamantane Ligand" Molecules 23, no. 3: 701. https://doi.org/10.3390/molecules23030701
APA StyleKhanfar, M. A., Jaber, A. M., AlDamen, M. A., & Al-Qawasmeh, R. A. (2018). Synthesis, Characterization, Crystal Structure, and DFT Study of a New Square Planar Cu(II) Complex Containing Bulky Adamantane Ligand. Molecules, 23(3), 701. https://doi.org/10.3390/molecules23030701