Huberine, a New Canthin-6-One Alkaloid from the Bark of Picrolemma huberi
Abstract
:1. Introduction
2. Results and Discussion
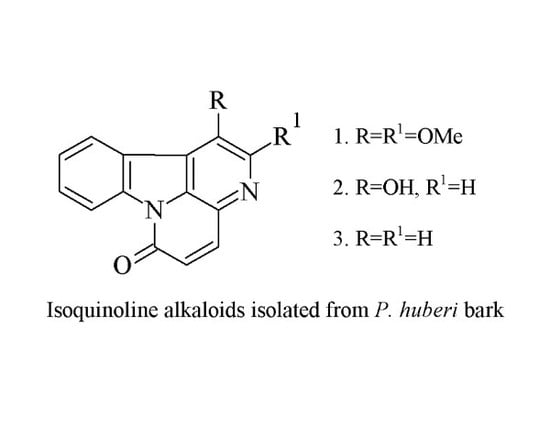
2.1. Identification of Isolated Compounds
2.2. Antiplasmodial Activity In Vitro
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohmoto, T.; Koike, K. The Alkaloids; Brossi, A., Ed.; Academic: New York, NY, USA, 1989; Volume 36, pp. 135–170. [Google Scholar]
- Tischler, M.; Cardellina, J.H., II; Boyd, M.R.; Cragg, G.M. Cytotoxic quassinoids from Cedronia granatensis. J. Nat. Prod. 1992, 55, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Fo, E.R.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F. Canthin-6-one alkaloids from Picrolemma granatensis. Phytochemistry 1992, 31, 2499–2501. [Google Scholar] [CrossRef]
- Rodrigues-Filho, E.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F. Quassinoids and tetranortriterpenoids from Picrolemma granatensis. Phytochemistry 1993, 34, 501–504. [Google Scholar] [CrossRef]
- Fo, E.R.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F.; Zukerman-Schpector, J.; Corrêa de Lima, R.M.O.; Nascimento, S.C.; Thomas, W. Protolimonoids and quassinoids from Picrolemma granatensis. Phytochemistry 1996, 43, 857–862. [Google Scholar] [CrossRef]
- Haynes, H.F.; Nelson, E.R.; Price, J.R. Alkaloids of the Australian Rutaceae: Pentaceras australis Hook. FI Isolation of the alkaloids and identification of canthin-6-one. Aust. J. Chem. 1952, 5, 387–400. [Google Scholar] [CrossRef]
- Crespi-Perellino, N.; Guicciardi, A.; Malyszko, G.; Minghetti, A. Biosynthetic relationship between indole alkaloids produced by cell cultures of Ailanthus altissima. J. Nat. Prod. 1986, 49, 814–822. [Google Scholar] [CrossRef]
- Aragozzini, F.; Maconi, E.; Gualandris, R. Evidence for involvement of ketoglutarate in the biosynthesis of canthin-6-one from cell cultures of Ailanthus altissima. Plant Cell Rep. 1988, 7, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Hay, C.A.; Roberts, M.F.; Phillipson, J.D. Studies on Ailanthus altissima cell suspension cultures. Plant Cell Rep. 1986, 5, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Showalter, H.D.H. Progress in the synthesis of canthine alkaloids and ring-truncated congeners. J. Nat. Prod. 2013, 76, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, T.; Koike, K.; Sakamoto, Y. Studies on the constituents of Ailanthus altissima Swingle. II. Alkaloidal constituents. Chem. Pharm. Bull. 1981, 29, 390–395. [Google Scholar] [CrossRef]
- Suroor, A.K.; Shamsuddin, K.M. 1-Hydroxycanthin-6-one, an alkaloid from Ailanthus giraldii. Phytochemistry 1981, 20, 2062–2063. [Google Scholar]
- Ohmoto, T.; Koike, K. Studies on the Constituents of Ailanthus altissima SWINGLE.III. The Alkaloidal Constituents. Chem. Pharm. Bull. 1984, 32, 170–173. [Google Scholar] [CrossRef]
- Nafiah, M.A.; Mukhtar, M.R.; Omar, H.; Ahmad, K.; Morita, H.; Litaudon, M.; Awang, K.; Hadi, A.H. N-Cyanomethylnorboldine: A New Aporphine Isolated from Alseodaphneperakensis (Lauraceae). Molecules 2011, 16, 3402–3409. [Google Scholar] [CrossRef] [PubMed]
- Pabón, A.; Ramirez, O.; Rios, A.; López, E.; De Las Salas, B.; Cardona, F.; Blair, S. Antiplasmodial and Cytotoxic Activity of Raw Plant extracts as reported by knowledgeable indigenous people of the amazon region (Vaupés Medio in Colombia). Plant Med. 2016, 82, 717–722. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2 and 3 are available from the authors. |

| Position | 1H (ppm), J (Hz) | 13C (ppm) | COSY Coupling | HMBC Coupling(2,3J) |
|---|---|---|---|---|
| 1 | 141.3 | OCH3 | ||
| 2 | 155.1 | OCH3 | ||
| 4 | 7.83(d, 9.6) | 138.2 | H-5 | H-5 |
| 5 | 6.82(d, 9.6) | 125.6 | H-4 | |
| 6 | 160.1 | H-4 | ||
| 8 | 8.67(d, 8.1) | 117.4 | H-9 | H-9 |
| 9 | 7.66 (t, 7.6) | 130.4 | H-8, H-10 | H-11 |
| 10 | 7.51 (t, 7.7) | 125.8 | H-9, H-11 | |
| 11 | 8.22(d, 7.6) | 124.9 | H-10 | H-9 |
| 12 | 123.5 | H-10 | ||
| 13 | 140.1 | H-9, H-11 | ||
| 14 | 130.3 | |||
| 15 | 130.3 | H-4 | ||
| 16 | 126.4 | H-5 | ||
| OCH3 | 4.15 (s) | 54.7 | ||
| OCH3 | 4.20 (s) | 61.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, C.; Pastrana, M.; Ríos, A.; Cogollo, A.; Pabón, A. Huberine, a New Canthin-6-One Alkaloid from the Bark of Picrolemma huberi. Molecules 2018, 23, 934. https://doi.org/10.3390/molecules23040934
López C, Pastrana M, Ríos A, Cogollo A, Pabón A. Huberine, a New Canthin-6-One Alkaloid from the Bark of Picrolemma huberi. Molecules. 2018; 23(4):934. https://doi.org/10.3390/molecules23040934
Chicago/Turabian StyleLópez, Carlos, Manuel Pastrana, Alexandra Ríos, Alvaro Cogollo, and Adriana Pabón. 2018. "Huberine, a New Canthin-6-One Alkaloid from the Bark of Picrolemma huberi" Molecules 23, no. 4: 934. https://doi.org/10.3390/molecules23040934
APA StyleLópez, C., Pastrana, M., Ríos, A., Cogollo, A., & Pabón, A. (2018). Huberine, a New Canthin-6-One Alkaloid from the Bark of Picrolemma huberi. Molecules, 23(4), 934. https://doi.org/10.3390/molecules23040934