In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates for the Synthesis of Aryldihydronaphthalene Derivatives
Abstract
:1. Introduction
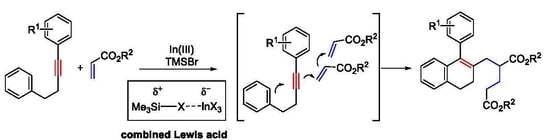
2. Results
2.1. Preliminary Result of In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates
2.2. Optimization of In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates
2.3. Substrate Scope of In(III)-TMSBr-Catalysed Cascade Reaction of Diarylalkynes with Acrylates
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for In(III)-TMSBr-Catalysed Cascade Reaction of Diarylalkyne with Acrylates
4.3. Product Characterization
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- James, M.J.; O’Brien, P.; Taylor, R.J.K.; Unsworth, W.P. Synthesis of spirocyclic indolenines. Chem. Eur. J. 2016, 22, 2856–2881. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-Y.; Chen, S.-L.; Yang, M.-H.; Wu, J.; Sinkkonen, J.; Zou, K. An update on lignans: Natural products and synthesis. Nat. Prod. Rep. 2009, 26, 1251–1292. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Reinhold, A.R.; Rosati, R.L.; Liu, K.K.C. Enzyme-catalyzed asymmetric deacylation for the preparation of Lasofoxifene (CP-336156), a selective estrogen receptor modulator. Org. Lett. 2000, 2, 4025–4027. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.-U.; Malik, A.; Anis, I.; Khan, S.B.; Ahmed, E.; Ahmed, Z.; Nawas, S.A.; Choudhary, M.I. Enzymes inhibiting lignans from Vitex Negundo. Chem. Pharm. Bull. 2004, 52, 1269–1272. [Google Scholar] [CrossRef]
- Kocsis, L.S.; Brummond, K.M. Intramolecular dehydro-Diels–Alder reaction affords selective entry to arylnaphthalene or aryldihydronaphthalene lignans. Org. Lett. 2014, 16, 4158–4161. [Google Scholar] [CrossRef] [PubMed]
- Magoulas, G.E.; Papaioannou, D. Bioinspired syntheses of dimeric hydroxycinnamic acids (lignans) and hybrids, using phenol oxidative coupling as key reaction, and medicinal significance thereof. Molecules 2014, 19, 19769–19835. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; Siqueira, F.A.; Pedrozo, E.C.; Vieira, F.Y.M.; Doriguetto, A.C. Iodine(III)-promoted ring contraction of 1,2-dihydronaphthalenes: Adiastereoselective total synthesis of (±)-Indatraline. Org. Lett. 2007, 9, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Scribner, A.W.; Haroutounian, S.A.; Carlson, K.E.; Katzenellenbogen, J.A. 1-Aryl-2-pyridyl-3,4-dihydronaphthalenes: photofluorogenic ligands for the estrogen receptor. J. Org. Chem. 1997, 62, 1043–1057. [Google Scholar] [CrossRef]
- Marieke, V.; Iris, A.; Christiane, S.; Ursula, M.-V.; Klaus, B.; Sandrine, M.-O.; Rolf, W.H. Synthesis and evaluation of heteroaryl-substituted dihydronaphthalenes and indenes: Potent and selective inhibitors of aldosterone synthase (CYP11B2) for the treatment of congestive heart failure and myocardial fibrosis. J. Med. Chem. 2006, 49, 2222–2231. [Google Scholar] [CrossRef]
- Bandini, M.; Emer, E.; Tommasi, S.; Umani-Ronchi, A. Innovative catalytic protocols for the ring-closing Friedel–Crafts-typealkylation and alkenylation of arenes. Eur. J. Org. Chem. 2006, 3527–3544. [Google Scholar] [CrossRef]
- Kitamura, T. Transition-metal-catalyzed hydroarylation reactions of alkynes through direct functionalization of C–H bonds: a convenient tool for organic synthesis. Eur. J. Org. Chem. 2009, 1111–1125. [Google Scholar] [CrossRef]
- Yamamoto, Y. Synthesis of heterocycles via transition-metal catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. [Google Scholar] [CrossRef] [PubMed]
- Namyslo, J.C.; Storsberg, J.; Klinge, J.; Gärtner, C.; Yao, M.-L.; Ocal, N.; Kaufmann, D.E. The hydroarylation reaction—Scope and limitations. Molecules 2010, 15, 3402–3410. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Lu, W.; Oyamada, J.; Kitamura, T.; Matsuda, K.; Irie, M.; Fujiwara, Y. Novel Pd(II)- and Pt(II)-catalyzed regio- and stereoselective trans-hydroarylation of alkynes by simple arenes. J. Am. Chem. Soc. 2000, 122, 7252–7263. [Google Scholar] [CrossRef]
- Jia, C.; Pao, D.; Oyamada, J.; Lu, W.; Kitamura, T.; Fujiwara, Y. Efficient activation of aromatic C-H bonds for addition to C-C multiple bonds. Science 2000, 287, 1992–1995. [Google Scholar] [CrossRef] [PubMed]
- Pastine, S.J.; Youn, S.W.; Sames, D. PtIV-catalyzed cyclization of arene−alkyne substrates via intramolecular electrophilic hydroarylation. Org. Lett. 2003, 5, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Takao, H.; Yadav, V.K.; Imagawa, H.; Sugihara, T. Mercuric triflate-(TMU)3-catalyzed cyclization of ω-arylalkyne leading to dihydronaphthalenes. Org. Lett. 2003, 5, 4563–4565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kozmin, S.A. Brønsted acid-promoted cyclizations of siloxyalkynes with arenes and alkenes. J. Am. Chem. Soc. 2004, 126, 10204–10205. [Google Scholar] [CrossRef] [PubMed]
- Nevado, C.; Echavarren, A.M. Intramolecular hydroarylation of alkynes catalyzed by platinum or gold: Mechanism and endo selectivity. Chem.–Eur. J. 2005, 11, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Barluenga, J.; Trincado, M.; Marco-Arias, M.; Ballesteros, A.; Rubio, E.; González, J.M. Intramolecular iodoarylation reaction of alkynes: easy access toderivatives of benzofused heterocycles. Chem. Commun. 2005, 2008–2010. [Google Scholar] [CrossRef] [PubMed]
- Gurtis, N.R.; Rassias, P.G.; Walker, A.J. A facile gold(I)-catalysed intramolecular alkyne hydroarylation approach to methyl 5-amino-2H-1-benzopyran-8-carboxylate derivatives. Tetrahedron Lett. 2008, 49, 6279. [Google Scholar] [CrossRef]
- Qiu, W.-W.; Surendra, K.; Yin, L.; Corey, E.J. Selective formation of six-membered oxa- and carbocycles by the In(III)-activated ring closure of acetylenic substrates. Org. Lett. 2011, 13, 5893–5895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, K.; Lan, S.; Zhang, L. Rapid access to chroman-3-ones through gold-catalyzed oxidation of propargyl aryl ethers. Angew. Chem. Int. Ed. 2012, 51, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Blesi, F.; Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. Gold versus silver catalyzed intramolecular hydroarylation reactions of [(3-arylprop-2-ynyl)oxy]benzene derivatives. Org. Biomol. Chem. 2012, 10, 9700–9708. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.; Mo, J.; Lee, P.H.; Gao, Z.; Kim, S. Synthesis of vinyl sulfides and vinylamines through catalytic intramolecular hydroarylation in the presence of FeCl3 and AgOTf. Eur. J. Org. Chem. 2013, 533–540. [Google Scholar] [CrossRef]
- Morán-Poladura, P.; Rubio, E.; González, J.M. Gold(I)-catalyzed hydroarylation reaction of aryl (3-iodoprop-2-yn-1-yl) ethers: synthesis of 3-iodo-2H-chromene derivatives. Beilstein J. Org. Chem. 2013, 9, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.; Park, S.; Park, Y.; Lee, K.; Hong, G.; Lee, P.H. Brønsted acid catalyzed intramolecular hydroarylation for the synthesis of cycloalkenyl selenides and tellurides. Eur. J. Org. Chem. 2013, 2672–2682. [Google Scholar] [CrossRef]
- Walkinshaw, A.J.; Xu, W.; Suero, M.G.; Gaunt, M.J. Copper-catalyzed carboarylation of alkynes via vinyl cations. J. Am. Chem. Soc. 2013, 135, 12532–12535. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Senda, K.; Senoo, M.; Hata, T.; Urabe, H. Rhodium-catalyzed intramolecular hydroarylation of 1-halo-1-alkynes: regioselective synthesis of semihydrogenated aromatic heterocycles. Chem. Eur. J. 2014, 20, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.-L.; Gong, T.-J.; Xiao, B.; Fu, Y. Copper-catalyzed endo-type trifluoromethyl arylation of alkynes. Chem. Commun. 2014, 50, 12915–12918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Zhang, W.-M.; Dai, J.-J.; Feng, Y.-S.; Xu, H.-J. Cu-catalyzed intramolecular hydroarylation of alkynes. RSC Adv. 2014, 4, 61706–61710. [Google Scholar] [CrossRef]
- Fu, L.; Niggemann, M. Calcium-catalyzed carboarylation of alkynes. Chem. Eur. J. 2015, 21, 6367–6370. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.J.; Lawson, J.R.; Fasano, V.; Ingleson, M.J. Formation of C(sp2)-boronate esters by borylative cyclization of alkynes using BCl3. Angew. Chem. Int. Ed. 2015, 54, 11245–11249. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Mou, T.; Feng, M.; Jiang, X. Utility of ligand effect in homogenous gold catalysis: enabling regiodivergent π-bond-activated cyclization. J. Am. Chem. Soc. 2016, 138, 5218–5221. [Google Scholar] [CrossRef] [PubMed]
- Lau, V.M.; Pfalzgraff, W.C.; Markland, T.E.; Kanan, M.W. Electrostatic control of regioselectivity in Au(I)-catalyzed hydroarylation. J. Am. Chem. Soc. 2017, 139, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Marañón, L.; Martínez, M.M.; Sarandeses, L.A.; Pérez Sestelo, J. Indium-catalyzed intramolecular hydroarylation ofaryl propargyl ethers. Org. Biomol. Chem. 2015, 13, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Marañón, L.; Sarandeses, L.A.; Martínez, M.M.; Pérez Sestelo, J. Sequential In-catalyzed intramolecular hydroarylation and Pd-catalyzed cross-coupling reactions using bromopropargyl aryl ethers and amines. Org. Chem. Front. 2017, 4, 500–505. [Google Scholar] [CrossRef]
- Li, C.J.; Chan, T.H. Organic synthesis using indium-mediated and catalyzed reactions in aqueous media. Tetrahedron 1999, 55, 11149–11176. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Wang, S.-Y.; Chok, Y.-K.; Xu, Y.-H.; Loh, T.-P. Organoindium reagents: the preparation and application in organic synthesis. Chem. Rev. 2013, 113, 271–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shen, L.; Shen, Z.-L.; Loh, T.-P. Transition metal-catalyzed cross-coupling reactions using organoindium reagents. Chem. Soc. Rev. 2017, 46, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Ito, T.; Yasuda, M.; Baba, A. Indium(III) chloride/chlorotrimethylsilane as a highly active Lewis acid catalyst system for the Sakurai-Hosomi reaction. Eur. J. Org. Chem. 2002, 1578–1581. [Google Scholar] [CrossRef]
- Saito, T.; Nishimoto, Y.; Yasuda, M.; Baba, A. Direct coupling reaction between alcohols and silyl compounds: enhancement of Lewis acidity of Me3SiBr using InCl3. J. Org. Chem. 2006, 71, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Lee, K.; Sung, S.-Y.; Chang, S. The catalytic Sakurai reaction. J. Org. Chem. 2001, 66, 8646–8649. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.; Corey, E.J. Diiodoindium(III) cation, InI2+, a potent yneophile. Generation and application to cationic cyclization by selective ϖ-activation of C≡C. J. Am. Chem. Soc. 2014, 136, 10918–10920. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Loh, T.-P. Highly stereoselective Prins cyclization of (Z)-and (E)-γ-brominated homoallylic alcohols to 2,4,5,6-tetrasubstituted tetrahydropyrans. Org. Lett. 2007, 9, 2063–2066. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.H.; Liu, F.; Loh, T.-P. Stereoelectronic versus steric tuning in the Prins cyclization Reaction: synthesis of 2,6-trans pyranyl motifs. Org. Lett. 2009, 11, 1741–1743. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhao, K.; Doitomi, K.; Ganguly, R.; Li, Y.-X.; Shen, Z.-L.; Hirao, H.; Loh, T.-P. Lewis acid-catalyzed selective [2 + 2]-cycloaddition and dearomatizing cascade reaction of aryl alkynes with acrylates. J. Am. Chem. Soc. 2017, 139, 13570–13578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-F.; Loh, T.-P. Acid-catalyzed intramolecular [2 + 2] cycloaddition of ene-allenones: facile access to bicyclo[n.2.0] frameworks. Angew. Chem. Int. Ed. 2009, 48, 7232–7235. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lai, Y.-C.; Zhao, Y.J.; Wong, Y.-H.; Shen, Z.L.; Loh, T.-P. Synthesis of 3-oxaterpenoids and its application in the total synthesis of (±)-moluccanic acid methyl ester. Angew. Chem. Int. Ed. 2012, 51, 10619–10623. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3aa–ja and 3ab–ae are available from the authors. |







| Entry | Catalyst | Solvent | TMSX | Yield (%) b |
|---|---|---|---|---|
| 1 | InBr3 | CH2Cl2 | TMSBr | 50 |
| 2 | InBr3 | CH2Cl2 | - | 0 |
| 3 | - | CH2Cl2 | TMSBr | 0 c |
| 4 | InCl3 | CH2Cl2 | TMSBr | 14 |
| 5 | InI3 | CH2Cl2 | TMSBr | 47 |
| 6 | In(OTf)3 | CH2Cl2 | TMSBr | 37 |
| 7 | In(tfacac)3 | CH2Cl2 | TMSBr | 61 |
| 8 | In(acac)3 | CH2Cl2 | TMSBr | 0 |
| 9 | AlBr3 | CH2Cl2 | TMSBr | 0 |
| 10 | ZnCl2 | CH2Cl2 | TMSBr | 0 |
| 11 | In(tfacac)3 | ClCH2CH2Cl | TMSBr | 70 |
| 12 | In(tfacac)3 | ClCH2CH2Cl | TMSCl | <5 |
| 13 | In(tfacac)3 | ClCH2CH2Cl | TMSBr | 60 c |
| 14 | In(tfacac)3 | ClCH2CH2Cl | TMSBr | 57 d |
| 15 | In(tfacac)3 | ClCH2CH2Cl | TMSBr | 33 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.-C.; Zhang, W.-W.; Shen, L.; Shen, Z.-L.; Loh, T.-P. In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates for the Synthesis of Aryldihydronaphthalene Derivatives. Molecules 2018, 23, 979. https://doi.org/10.3390/molecules23040979
Zhang Q-C, Zhang W-W, Shen L, Shen Z-L, Loh T-P. In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates for the Synthesis of Aryldihydronaphthalene Derivatives. Molecules. 2018; 23(4):979. https://doi.org/10.3390/molecules23040979
Chicago/Turabian StyleZhang, Qiu-Chi, Wen-Wei Zhang, Liang Shen, Zhi-Liang Shen, and Teck-Peng Loh. 2018. "In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates for the Synthesis of Aryldihydronaphthalene Derivatives" Molecules 23, no. 4: 979. https://doi.org/10.3390/molecules23040979
APA StyleZhang, Q. -C., Zhang, W. -W., Shen, L., Shen, Z. -L., & Loh, T. -P. (2018). In(III)-TMSBr-Catalyzed Cascade Reaction of Diarylalkynes with Acrylates for the Synthesis of Aryldihydronaphthalene Derivatives. Molecules, 23(4), 979. https://doi.org/10.3390/molecules23040979