Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule
Abstract
:1. Introduction
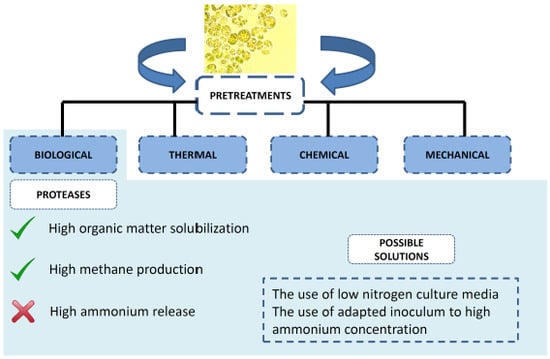
2. Pretreatment of Microalgae Biomass to Improve Biogas Production
2.1. High Energy Demanding Pretreatments
2.2. Low Energy Demanding Pretreatments
3. Biological Approach to Enhance Biogas Production: Enzymatic Pretreatment
3.1. Carbohydrases
3.2. Lipases
3.3. Proteases
4. Biomass Proteins in Anaerobic Digestion of Microalgae
4.1. The Relevance of Microalgae Proteins in the Hydrolysis Stage of Anaerobic Digestion
4.2. The Relevance of Microalgae Proteins in the Methanogenesis Stage of Anaerobic Digestion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Henze, M.; van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment; IWA Publishing: London, UK, 2008. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Addy, M.M.; Zhao, J.; Cheng, Y.; Cheng, S.; Mu, D.; Liu, Y.; Ding, R.; Chen, P.; Ruan, R. Comprehensive techno-economic analysis of wastewater-based algal biofuel production: A case study. Bioresour. Technol. 2016, 211, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Gutiérrez, R.; Uggetti, E.; Garfí, M.; García, J.; Ferrer, I. Towards energy neutral microalgae-based wastewater treatment plants. Algal Res. 2017, 28, 235–243. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Energy Balance of Biogas Production from microalgae: Development of an energy and mass balance model. Curr. Biotechnol. 2015, 4, 554–567. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Montero, N.; Ferrer, I.; Acién, F.G.; Gómez, C.; Garfí, M. Life cycle assessment of high rate algal ponds for wastewater treatment and resource recovery. Sci. Total Environ. 2018, 622, 1118–1130. [Google Scholar] [CrossRef]
- Muñoz, R.; Gonzalez-Fernandez, C. Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing Series in Energy; Elsevier Science: New York, NY, USA, 2017. [Google Scholar]
- Ometto, F.; Quiroga, G.; Psenicka, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Re/Views Environ. Sci. Bio/Technol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Carbohydrate-enriched cyanobacterial biomass as feedstock for bio-methane production through anaerobic digestion. Fuel 2013, 111, 872–879. [Google Scholar] [CrossRef]
- Yu, W.-L.; Ansari, W.; Schoepp, N.G.; Hannon, M.J.; Mayfield, S.P.; Burkart, M.D. Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb. Cell Fact. 2011, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Fábregas, J.; Maseda, A.; Domínguez, A.; Otero, A. The cell composition of Nannochloropsis sp. changes under different irradiances in semicontinuous culture. World J. Microbiol. Biotechnol. 2004, 20, 31–35. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [PubMed]
- Demuez, M.; Mahdy, A.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol. Bioeng. 2015, 112, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, J.W.; Versteegh, G.J.M.; van Bergen, P.F. Biomacromolecules of algae and plants and their fossil analogues. In Plants and Climate Change; Rozema, J., Aerts, R., Cornelissen, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 209–233. [Google Scholar]
- Kodner, R.B.; Summons, R.E.; Knoll, A.H. Phylogenetic investigation of the aliphatic, non-hydrolyzable biopolymer algaenan, with a focus on green algae. Org. Geochem. 2009, 40, 854–862. [Google Scholar] [CrossRef] [Green Version]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Klassen, V.; Blifernez-Klassen, O.; Wobbe, L.; Schluter, A.; Kruse, O.; Mussgnug, J.H. Efficiency and biotechnological aspects of biogas production from microalgal substrates. J. Biotechnol. 2016, 234, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, C.; Sialve, B.; Molinuevo-Salces, B. Anaerobic digestion of microalgal biomass: Challenges, opportunities and research needs. Bioresour. Technol. 2015, 198, 896–906. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.-P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuels Bioprod. Biorefin. 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Passos, F.; Ferrer, I.; Vicent, T.; Blánquez, P. Enzymatic pretreatment of microalgae using fungal broth from Trametes versicolor and commercial laccase for improved biogas production. Algal Res. 2016, 19, 184–188. [Google Scholar] [CrossRef]
- Cordova, O.; Passos, F.; Chamy, R. Physical pretreatment methods for improving microalgae anaerobic biodegradability. Appl. Biochem. Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Negi, S.; Binod, P.; Larroche, C. Pretreatment of Biomass: Processes and Technologies; Elsevier Science: New York, NY, USA, 2014. [Google Scholar]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Microalgae conversion to biogas: Thermal pretreatment contribution on net energy production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Méndez, L.; Ballesteros, M.; Tomás-Pejó, E. Hydrothermal Processing of Microalgae. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass; Ruiz, H.A., Hedegaard Thomsen, M., Trajano, H.L., Eds.; Springer: Cham, The Netherlands, 2017. [Google Scholar]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal pretreatment of microalgae for biomethane production: Experimental studies, kinetics and energy analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Biomethane production using fresh and thermally pretreated Chlorella vulgaris biomass: A comparison of batch and semi-continuous feeding mode. Ecol. Eng. 2015, 84, 273–277. [Google Scholar] [CrossRef]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical methane potential of microalgae: Influence of substrate to inoculum ratio, biomass concentration and pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.; Mahdy, A.; Demuez, M.; Ballesteros, M.; González-Fernández, C. Effect of high pressure thermal pretreatment on Chlorella vulgaris biomass: Organic matter solubilisation and biochemical methane potential. Fuel 2014, 117, 674–679. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Autohydrolysis and alkaline pretreatment effect on Chlorella vulgaris and Scenedesmus sp. methane production. Energy 2014, 78, 48–52. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Barragán-Trinidad, M.; Buitrón, G. Biological pretreatments of microalgal biomass for gaseous biofuel production and the potential use of rumen microorganisms: A review. Algal Res. 2016, 18, 341–351. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energy Convers. Manag. 2014, 85, 551–557. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Protease pretreated Chlorella vulgaris biomass bioconversion to methane via semi-continuous anaerobic digestion. Fuel 2015, 158, 35–41. [Google Scholar] [CrossRef]
- Mahdy, A.; Ballesteros, M.; González-Fernández, C. Enzymatic pretreatment of Chlorella vulgaris for biogas production: Influence of urban wastewater as a sole nutrient source on macromolecular profile and biocatalyst efficiency. Bioresour. Technol. 2016, 199, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, A.; Mendez, L.; Tomás-Pejó, E.; del Mar Morales, M.; Ballesteros, M.; González-Fernández, C. Influence of enzymatic hydrolysis on the biochemical methane potential of Chlorella vulgaris and Scenedesmus sp. J. Chem. Technol. Biotechnol. 2016, 91, 1299–1305. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of hydrothermal pretreatment on microalgal biomass anaerobic digestion and bioenergy production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Alaswad, A.; Mooney, J.; Prescott, T.; Olabi, A.G. Pre-treatment techniques used for anaerobic digestion of algae. Fuel Process. Technol. 2015, 138, 765–779. [Google Scholar] [CrossRef]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Martínez, N.; Callejas, N.; Morais, E.G.; Vieira Costa, J.A.; Jachmanián, I.; Vieitez, I. Obtaining biodiesel from microalgae oil using ultrasound-assisted in-situ alkaline transesterification. Fuel 2017, 202, 512–519. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Cheng, C.-L.; Ho, S.-H.; Chang, J.-S.; Ren, N. Enhancing bio-butanol production from biomass of Chlorella vulgaris JSC-6 with sequential alkali pretreatment and acid hydrolysis. Bioresour. Technol. 2016, 200, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Carrère, H.; Garfí, M.; Ferrer, I. Enhancement of microalgae anaerobic digestion by thermo-alkaline pretreatment with lime (CaO). Algal Res. 2017, 24, 199–206. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Timmers, R.A.; Ballesteros, M.; González-Fernández, C. Enhancing methane production of Chlorella vulgaris via thermochemical pretreatments. Bioresour. Technol. 2013, 149, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Chng, L.M.; Lee, K.T.; Chan, D.J.C. Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers. Manag. 2017, 141, 410–419. [Google Scholar] [CrossRef]
- Khan, M.I.; Lee, M.G.; Shin, J.H.; Kim, J.D. Pretreatment optimization of the biomass of Microcystis aeruginosa for efficient bioethanol production. AMB Express 2017, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Subbulakshmi, P.; Rajesh Banu, J.; Gobi, M.; Tae Yeom, I. Enhancement of biogas production from microalgal biomass through cellulolytic bacterial pretreatment. Bioresour. Technol. 2017, 233, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ciudad, G.; Rubilar, O.; Azócar, L.; Toro, C.; Cea, M.; Torres, Á.; Ribera, A.; Navia, R. Performance of an enzymatic extract in Botrycoccus braunii cell wall disruption. J. Biosci. Bioeng. 2014, 117, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Hidalgo, C.; Zapata, M.; Jeison, D.; Riquelme, C.; Rivas, M. Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl. Environ. Microbiol. 2014, 80, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving biogas production from microalgae by enzymatic pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fan, X.; Katukuri, N.R.; Yuan, X.; Wang, F.; Guo, R.-B. Enhanced methane production from microalgal biomass by anaerobic bio-pretreatment. Bioresour. Technol. 2016, 204, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Arenas, E.G.; Rodriguez Palacio, M.C.; Juantorena, A.U.; Fernando, S.E.L.; Sebastian, P.J. Microalgae as a potential source for biodiesel production: Techniques, methods, and other challenges. Int. J. Energy Res. 2017, 41, 761–789. [Google Scholar] [CrossRef]
- Aydin, S. Enhancement of microbial diversity and methane yield by bacterial bioaugmentation through the anaerobic digestion of Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2016, 100, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, R.; Incharoensakdi, A. MgO-Fe3O4 linked cellulase enzyme complex improves the hydrolysis of cellulose from Chlorella sp. CYB2. Biochem. Eng. J. 2017, 122, 22–30. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Fan, L.; Linklater, A.; Zheng, B.; Jiang, D.; Qin, W. Enzymes produced by biomass-degrading bacteria can efficiently hydrolyze algal cell walls and facilitate lipid extraction. Renew. Energy 2017, 109, 195–201. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Palatsi, J.; Laureni, M.; Andrés, M.V.; Flotats, X.; Nielsen, H.B.; Angelidaki, I. Strategies for recovering inhibition caused by long chain fatty acids on anaerobic thermophilic biogas reactors. Bioresour. Technol. 2009, 100, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Solé, M.; García, J.; Ferrer, I. Biogas production from microalgae grown in wastewater: Effect of microwave pretreatment. Appl. Energy 2013, 108, 168–175. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Holm-Nielsen, J.-B.; Poulsen, M.; Boelsmand, J.E. Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew. Energy 2013, 50, 476–480. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Gerken, H.G.; Donohoe, B.; Knoshaug, E.P. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 2013, 237, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA anaerobic digestion model no 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [PubMed]
- Miao, H.; Lu, M.; Zhao, M.; Huang, Z.; Ren, H.; Yan, Q.; Ruan, W. Enhancement of Taihu blue algae anaerobic digestion efficiency by natural storage. Bioresour. Technol. 2013, 149, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A. Mini review: Update on bioaugmentation in anaerobic processes for biogas production. Anaerobe 2017, 46, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fermoso, F.G.; Bartacek, J.; Jansen, S.; Lens, P.N.L. Metal supplementation to UASB bioreactors: From cell-metal interactions to full-scale application. Sci. Total Environ. 2009, 407, 3652–3667. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gomec, C.Y.; Ahn, Y.; Speece, R.E. Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environ. Technol. 2003, 24, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Cabanelas, I.T.D.; Arbib, Z.; Chinalia, F.A.; Souza, C.O.; Perales, J.A.; Almeida, P.F.; Druzian, J.I.; Nascimento, I.A. From waste to energy: Microalgae production in wastewater and glycerol. Appl. Energy 2013, 109, 283–290. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia Inhibition in Anaerobic Digestion: A Review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Illman, A.M.; Scragg, A.H.; Shales, S.W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 2000, 27, 631–635. [Google Scholar] [CrossRef]
- An, M.; Wang, Y.; Liu, F.; Qi, X.; Zheng, Z.; Ye, N.; Sun, C.; Miao, J. Biomass, nutrient uptake and fatty acid composition of Chlamydomonas sp. ICE-L in response to different nitrogen sources. Acta Oceanol. Sin. 2017, 36, 105–110. [Google Scholar] [CrossRef]
- Nakakubo, R.; Møller, H.B.; Nielsen, A.M.; Matsuda, J. Ammonia inhibition of methanogenesis and identification of process indicators during anaerobic digestion. Environ. Eng. Sci. 2008, 25, 1487–1496. [Google Scholar] [CrossRef]
- Mahdy, A.; Fotidis, I.A.; Mancini, E.; Ballesteros, M.; Gonzalez-Fernandez, C.; Angelidaki, I. Ammonia tolerant inocula provide a good base for anaerobic digestion of microalgae in third generation biogas process. Bioresour. Technol. 2017, 225, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed]


| High Demanding Energy Pretreatments | Operation Mode | Biomass | Conditions | Methane Yield Increase | References |
| Thermal | Batch | Scenedesmus sp. | 75 °C for 10 h 95 °C for 10 h | 58% 69% | [29,30,31] |
| Batch | Scenedesmus sp. | 80 °C for 15 min | 60% | [32] | |
| Batch | Chlorella sp. | 70 °C for 30 min 90 °C for 30 min | 37%48% | [33] | |
| Batch | Stigeoclonium sp. Monoraphidium sp and Nitzschia | 130 °C for 15–30 min | 28% | [31] | |
| Semi-continuous | Chlorella sp. | 120 °C40 min | 1.5-fold | [34] | |
| Mechanical | Batch | Scenedesmus sp. | 128.9 KJ/g TS for 30 min | 87% | [32] |
| Batch | Monoraphidium sp. and Stigeoclonium sp. | 26.7 KJ/g TS for 30 min | 85% | [31] | |
| Batch | Mixture of microalgae biomass | 10; 27; 40; 57 KJ/g TS | 6-24% | [35] | |
| Chemical | Batch | Chlorella sp. and Scenedesmus sp. | CaO (4 and 10% w/w) at 25, 55 and 72 °C | 25% | [36] |
| Batch | Chlorella sp. | 4 M H2SO4 at 120 °C for 20–40 min | 72.5% | [37] | |
| Low Demanding Energy Pretreatments | Biomass | Solubilization | Methane Yield | References | |
| Proteases | Batch | C. reinhardtii C. vulgaris | 86-96% for both biomasses | 51% in Chlorella biomass 7% C. reindhartii | [38] |
| Batch | Scenedesmus sp. | 30% | 1.53-fold | [39] | |
| Semi-continuous | C.vulgaris | 47% | 2.6-fold | [39] | |
| Semi-continuous | C. vulgaris | 54% | 5 and 6.3-fold (OLR= 1.5 g/L d and OLR= 3 g/L d ) | [40] | |
| Carbohydrases | Batch | C. vulgaris and Scenedesmus sp. | 84% 36% | 1.2-fold | [41] |
| Amino Acid | Formula | HAc | HProp | HBu | HVa | IN | IC | Other | H2 | ATP |
|---|---|---|---|---|---|---|---|---|---|---|
| Arginine | C6H14O2N4 | 0.5 | 0.5 | 0 | 0.5 | 4 | 1 | 0 | −1 | 1 |
| Histidine | C6H9O2N3 | 1 | 0 | 0.5 | 0 | 3 | 1 | 1 | 0 | 2 |
| Lysine | C6H14O2N2 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 |
| Tyrosine | C9H11O3N | 1 | 0 | 0 | 0 | 1 | 1 | 0.882 | 1 | 1 |
| Tryptophan | C11H12O3N | 0 | 0 | 0 | 0 | 1 | 1 | 1.471 | 2 | 1 |
| Phenylalanine | C9H11O2N | 0 | 0 | 0 | 0 | 1 | 1 | 1.176 | 2 | 1 |
| Cysteine | C3H6O2NS | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Methionine | C5H11O2NS | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Threonine | C4H9O3N | 1 | 0 | 0.5 | 0 | 1 | 0 | 0 | −1 | 1 |
| Serine | C3H7O3N | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Leucine/Isoleucine | C6H13O2N | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 1 |
| Valine | C5H11O2N | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 2 | 1 |
| Glutamine | C5H9O4N | 1 | 0 | 0.5 | 0 | 1 | 1 | 0 | 0 | 2 |
| Aspartate | C4H7O4N | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 2 |
| Glycine | C2H5O2N | 1 | 0 | 0 | 0 | 1 | 0 | 0 | −1 | 0 |
| Alanine | C3H7O2N | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 1 |
| Proline | C5H9O2N | 0.5 | 0.5 | 0 | 0.5 | 1 | 0 | 0 | −1 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdalena, J.A.; Ballesteros, M.; González-Fernandez, C. Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule. Molecules 2018, 23, 1098. https://doi.org/10.3390/molecules23051098
Magdalena JA, Ballesteros M, González-Fernandez C. Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule. Molecules. 2018; 23(5):1098. https://doi.org/10.3390/molecules23051098
Chicago/Turabian StyleMagdalena, Jose Antonio, Mercedes Ballesteros, and Cristina González-Fernandez. 2018. "Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule" Molecules 23, no. 5: 1098. https://doi.org/10.3390/molecules23051098
APA StyleMagdalena, J. A., Ballesteros, M., & González-Fernandez, C. (2018). Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule. Molecules, 23(5), 1098. https://doi.org/10.3390/molecules23051098