Subcritical Water-Carbon Dioxide Pretreatment of Oil Palm Mesocarp Fiber for Xylooligosaccharide and Glucose Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Compositional Analysis
2.2. Physico-Chemical Properties of Untreated and Pretreated Samples
2.2.1. Solids Recovery
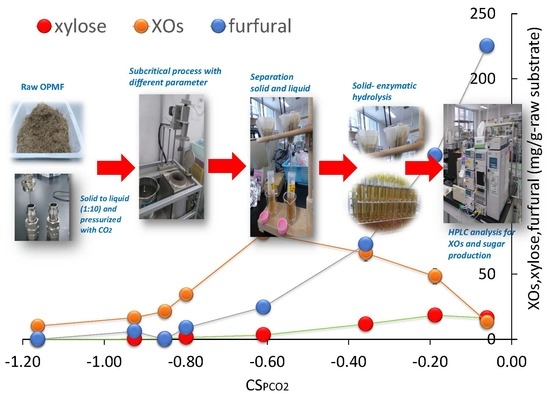
2.2.2. Xylooligosaccharide Content in the Pretreatment Liquids
2.2.3. Monomeric Sugars, Acids, Furans and Tannic Acids Content in the Pretreatment Liquids
2.2.4. pH of the Pretreatment Liquids
2.3. Types of XOs Produced
2.4. Enzymatic Hydrolysis of Pretreated Solids
2.5. Cellulose Crystallinity Index
2.6. Specific Surface Area
2.7. SEM Analysis
3. Materials and Methods
3.1. Raw Material Preparation
3.2. Chemical Compositional Analysis
3.3. Subcritical H2O and Subcritical H2O-CO2 Pretreatments
3.4. Combined Severity Factor
3.5. Determination of Monomeric and Total Monomeric Sugars from Pretreatment Liquids
3.6. Determination of Tannic Acid
3.7. Determination Degree of Polymerization (DP) of XOs
3.8. Enzymatic Hydrolysis
3.9. SEM, BET and CrI Analyses
- I002: The intensity at about 2θ = 22.2°
- Iam: The intensity at 2θ = 17.6°
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Abdullah, N.; Sulaiman, F. The oil palm wastes in Malaysia. In Biomass Now-Sustainable Growth and Use; InTech: Rijeka, Croatia, 2013; pp. 75–93. [Google Scholar]
- Iberahim, N.I.; Jahim, J.M.; Harun, S.; Nor, M.T.M.; Hassan, O. Sodium hydroxide pretreatment and enzymatic hydrolysis of oil palm mesocarp fiber. Int. J. Chem. Eng. Appl. Sci. 2013, 4, 101–105. [Google Scholar] [CrossRef]
- Nordin, N.I.A.A.; Ariffin, H.; Andou, Y.; Hassan, M.A.; Shirai, Y.; Nishida, H.; Ibrahim, N.A. Modification of oil palm mesocarp fiber characteristics using superheated steam treatment. Molecules 2013, 18, 9132–9146. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.R.; Hirata, S.; Fujimoto, S.; Hassan, M.A. Combined pretreatment with hot compressed water and wet disk milling opened up oil palm biomass structure resulting in enhanced enzymatic digestibility. Bioresour. Technol. 2015, 193, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Combined pretreatment using alkaline hydrothermal and ball milling to enhance enzymatic hydrolysis of oil palm mesocarp fiber. Bioresour. Technol. 2014, 169, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Garrote, G.; Domí, H.; Parajó, J.C. Autohydrolysis of corncob: Study of non-isothermal operation for xylooligosaccharide production. J. Food Eng. 2002, 52, 211–218. [Google Scholar] [CrossRef]
- Da Silva, S.P.M.; Morais, A.R.C.; Bogel-Łukasik, R. The CO2-assisted autohydrolysis of wheat straw. Green Chem. 2014, 16, 238–246. [Google Scholar] [CrossRef]
- Morais, A.R.; Mata, A.C.; Bogel-Lukasik, R. Integrated conversion of agroindustrial residue with high pressure CO2 within the biorefinery concept. Green Chem. 2014, 16, 4312–4322. [Google Scholar] [CrossRef]
- Van Walsum, G.P. Severity function describing the hydrolysis of xylan using carbonic acid. In Twenty-Second Symposium on Biotechnology for Fuels and Chemicals; Humana Press: New York, NY, USA, 2001; pp. 317–329. [Google Scholar]
- Relvas, F.M.; Morais, A.R.C.; Bogel-Lukasik, R. Kinetic modeling of hemicellulose-derived biomass hydrolysis under high pressure CO2–H2O mixture technology. J. Supercrit. Fluids 2015, 99, 95–102. [Google Scholar] [CrossRef]
- Fockink, D.H.; Morais, A.R.C.; Ramos, L.P.; Lukasik, R.M. Insight into the high-pressure CO2 pre-treatment of sugarcane bagasse for a delivery of upgradable sugars. Energy 2018, 151, 536–544. [Google Scholar] [CrossRef]
- Toscan, A.; Morais, A.R.C.; Paixão, S.M.; Alves, L.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. High-pressure carbon dioxide/water pre-treatment of sugarcane bagasse and elephant grass: Assessment of the effect of biomass composition on process efficiency. Bioresour. Technol. 2017, 224, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Peng, P.; Peng, F.; Xiao, X.; Xu, F.; Sun, R.C. Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food Chem. 2014, 156, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E.; Carvalheiro, F.; Moura, P.; Ballesteros, I.; Castro, E.; Gírio, F. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind. Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef]
- Bragatto, J.; Segato, F.; Squina, F.M. Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Ind. Crops Prod. 2013, 51, 123–129. [Google Scholar] [CrossRef]
- Ho, A.L.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Charalampopoulos, D.; Rastall, R.A. Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour. Technol. 2014, 152, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, O.; Erdogan, K.; Bostanci, S. Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr. Res. 2009, 344, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Effect of autohydrolysis and enzymatic treatment on oil palm (Elaeisguineensis Jacq.) frond fibres for xylose and xylooligosaccharides production. Bioresour. Technol. 2011, 102, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.R.; Hirata, S.; Fujimoto, S.; Ibrahim, I.; Hassan, M.A. Soluble inhibitors generated during hydrothermal pretreatment of oil palm mesocarp fiber suppressed the catalytic activity of Acremonium cellulase. Bioresour. Technol. 2016, 200, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kim, T.H.; Lee, Y.Y.; Chen, R.; Elander, R.T. Enzymatic production of xylooligosaccharides from corn stover and corncobs treated with aqueous ammonia. In Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals; Humana Press: New York, NY, USA, 2006; pp. 586–598. [Google Scholar]
- Nabarlatz, D.; Ebringerová, A.; Montané, D. Autohydrolysis of agricultural by-products for the production of xylo-oligosaccharides. Carbohydr. Polym. 2007, 69, 20–28. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ahring, B.K. A thermochemical pretreatment process to produce xylooligosaccharides (XOS), arabinooligosaccharides (AOS) and mannooligosaccharides (MOS) from lignocellulosic biomasses. Bioresour. Technol. 2012, 112, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wyman, C.E. Characterization of the degree of polymerization of xylooligomers produced by flow through hydrolysis of pure xylan and corn stover with water. Bioresour. Technol. 2008, 99, 5756–5762. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal treatment and enzymatic hydrolysis of Tamarix ramosissima: Evaluation of the process as a conversion method in a biorefinery concept. Bioresour. Technol. 2013, 135, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, by-Products, and Degradation Products in Liquid Fraction Process Samples. NREL/TP-510-42623, Laboratory Analytical Procedure (LAPs); National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Chen, M.H.; Bowman, M.J.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Autohydrolysis of Miscanthus x giganteus for the production of xylooligosaccharides (XOS): Kinetics, characterization and recovery. Bioresour. Technol. 2014, 155, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.K.; Jayapal, N.; Kolte, A.P.; Senani, S.; Sridhar, M.; Suresh, K.P.; Sampath, K.T. Enzymatic production of xylooligosaccharides from alkali solubilized xylan of natural grass (Sehima nervosum). Bioresour. Technol. 2012, 112, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.S.; Krishnan, C. Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT-Food Sci. Technol. 2016, 65, 237–245. [Google Scholar] [CrossRef]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Hsu, T.C.; Guo, G.L.; Chen, W.H.; Hwang, W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xu, F.; Li, S.; Ji, X.; Chen, S.; Zhang, D. Effect of SC-CO2 pretreatment in increasing rice straw biomass conversion. Biosyst. Eng. 2010, 106, 470–475. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Inoue, H.; Yano, S.; Endo, T.; Sakaki, T.; Sawayama, S. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels 2008, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P. Quantification of Tannin in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Ishiguro, M.; Endo, T. Addition of alkali to the hydrothermal- mechanochemical treatment of Eucalyptus enhances its enzymatic saccharification. Bioresour. Technol. 2014, 153, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. 1959, 29, 786–794. [Google Scholar] [CrossRef]
Sample Availability: Samples of the raw OPMF is available from the authors. |
 ) xylobiose, (
) xylobiose, (  ) xylotriose, (
) xylotriose, (  ) xylotetraose, (- - -) XOs yield.
) xylotetraose, (- - -) XOs yield.
 ) xylobiose, (
) xylobiose, (  ) xylotriose, (
) xylotriose, (  ) xylotetraose, (- - -) XOs yield.
) xylotetraose, (- - -) XOs yield.

| Chemical Component | Content (wt %) | |||
|---|---|---|---|---|
| Solvent extractives | 8.3 ± 0.4 a | 11.4 ± 0.2 b | 6.3 ± 0.51 a | - |
| Cellulose | 23.6 ± 0.9 | 25.0 ± 1.7 | 28.8 ± 0.48 | 42.8 ± 0.69 |
| Hemicellulose | 22.3 ± 0.5 | 25.7 ± 3.3 | 25.3 ± 0.65 | 33.1 ± 2.01 |
| Klason Lignin | 28.2 ± 1.4 | 25.5 ± 0.5 | 28.9 ± 2.07 | 20.5 ± 3.44 |
| Ash | 5.8 ± 0.7 | 5.8 ± 0.2 | 2.6 ± 0.34 | 3.6 ± 0.74 |
| Reference | This study | Zakaria et al. [5] | Iberahim et al. [2] | Nordin et al. [3] |
| Reaction Conditions | Subcritical H2O | Subcritical CO2-H2O | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | 150 | 150 | 160 | 170 | 170 | 180 | 180 | 200 | ||||||||
| Time (min) | 60 | 60 | 40 | 20 | 40 | 40 | 60 | 20 | ||||||||
| Pressure (MPa) | 0 | 3 | 3 | 5 | 3 | 3 | 3 | 5 | ||||||||
| Log (Ro) | 3.25 | 3.25 | 3.37 | 3.36 | 3.66 | 3.96 | 4.13 | 4.25 | ||||||||
| pH (pretreated liquid) | 4.41 | 4.18 | 4.22 | 4.16 | 4.27 | 4.32 | 4.32 | 4.31 | ||||||||
| CSpCO2 | - | −0.93 | −0.85 | −0.80 | −0.61 | −0.36 | −0.19 | −0.06 | ||||||||
| Solid recovery (w/w %) | 83.29 | 84.14 | 82.41 | 80.37 | 70.22 | 63.25 | 68.45 | 62.56 | ||||||||
| Composition/yields | g/L | mg/g | g/L | mg/g | g/L | mg/g | g/L | mg/g | g/L | mg/g | g/L | mg/g | g/L | mg/g | g/L | mg/g |
| XOs * | 1.12 | 11.23 | 1.66 | 16.60 | 2.14 | 21.40 | 1.33 | 13.30 | 4.84 | 48.40 | 6.62 | 66.20 | 8.16 | 81.60 | 3.45 | 34.52 |
| Xylose | 0 | 0.05 | 0.03 | 0.26 | 0.05 | 0.50 | 0.15 | 1.50 | 0.33 | 3.30 | 1.17 | 11.70 | 1.85 | 18.50 | 1.64 | 16.40 |
| Glucose | 0.19 | 1.92 | 0.14 | 1.40 | 0.12 | 1.20 | 0.19 | 1.90 | 0.15 | 1.50 | 0.30 | 3.00 | 0.20 | 2.00 | 0.23 | 2.30 |
| Arabinose | 0.54 | 5.41 | 0.51 | 5.10 | 0.57 | 5.70 | 0.68 | 6.80 | 0.73 | 7.30 | 0.39 | 3.90 | 0.31 | 3.10 | 0.16 | 1.60 |
| Acetic acid | 4.07 | 40.70 | 3.36 | 33.60 | 3.78 | 37.80 | 4.94 | 49.40 | 14.73 | 147.30 | 22.59 | 225.90 | 32.33 | 323.30 | 38.16 | 381.60 |
| Furfural | 0 | 0 | 0 | 0 | 0 | 0 | 0.88 | 8.80 | 2.47 | 24.70 | 7.30 | 73.00 | 14.13 | 141.30 | 22.53 | 225.30 |
| 5-HMF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.37 | 3.70 | 0.60 | 6.00 | 1.00 | 10.00 |
| Formic acid | 8.06 | 80.64 | 8.07 | 80.70 | 8.12 | 81.20 | 8.40 | 84.00 | 11.66 | 116.60 | 13.27 | 132.70 | 17.06 | 170.60 | 18.03 | 180.30 |
| Tannic acid | 0.51 | - | 0.37 | - | 0.54 | - | 0.21 | - | 0.77 | - | 1.09 | - | 1.07 | - | 1.72 | - |
| Treatment Conditions | Untreated OPMF | Subcritical H2O | Subcritical H2O-CO2 | ||
|---|---|---|---|---|---|
| Temperature (°C) | - | 150 | 150 | 170 | 190 |
| Time (min) | - | 60 | 180 | 40 | 60 |
| Pressure (MPa) | - | 0 | 5 | 3 | 3 |
| Log, Ro | - | 3.25 | 3.73 | 3.66 | 4.43 |
| CSPCO2 | - | −1.16 | −0.34 | −0.61 | 0.06 |
| pH | - | 4.41 | 4.18 | 4.27 | 4.43 |
| Solid recovery (%) | - | 85.75 | 73.73 | 70.22 | 61.88 |
| Cellulose (%) | 23.58 | 22.61 | 28.29 | 29.24 | 36.67 |
| Hemicellulose (%) | 22.34 | 17.94 | 12.14 | 12.31 | 3.14 |
| CrI (%) | 52.35 | 62.35 | 58.92 | 59.10 | 63.47 |
| SSA (m2 g−1) | 2.33 | 8.17 | 17.11 | 8.18 | 20.22 |
| Pore volume (cm3 g−1) | 0.01 | 0.04 | 0.08 | 0.04 | 0.01 |
| * Sugar yield (%) | |||||
| Glucose | 15.60 ± 7.5 | 31.83 ± 3.9 | 68.72 ± 11.0 | 70.26 ± 4.4 | 84.65 ± 2.5 |
| Xylose | 5.65 ± 0.6 | 16.99 ± 3.2 | 28.05 ± 2.8 | 29.96 ± 0 | 5.43 ± 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Zakaria, M.R.; Mohd Yusoff, M.Z.; Fujimoto, S.; Inoue, H.; Ariffin, H.; Hassan, M.A.; Shirai, Y. Subcritical Water-Carbon Dioxide Pretreatment of Oil Palm Mesocarp Fiber for Xylooligosaccharide and Glucose Production. Molecules 2018, 23, 1310. https://doi.org/10.3390/molecules23061310
Ahmad N, Zakaria MR, Mohd Yusoff MZ, Fujimoto S, Inoue H, Ariffin H, Hassan MA, Shirai Y. Subcritical Water-Carbon Dioxide Pretreatment of Oil Palm Mesocarp Fiber for Xylooligosaccharide and Glucose Production. Molecules. 2018; 23(6):1310. https://doi.org/10.3390/molecules23061310
Chicago/Turabian StyleAhmad, Norlailiza, Mohd Rafein Zakaria, Mohd Zulkhairi Mohd Yusoff, Shinji Fujimoto, Hiroyuki Inoue, Hidayah Ariffin, Mohd Ali Hassan, and Yoshihoto Shirai. 2018. "Subcritical Water-Carbon Dioxide Pretreatment of Oil Palm Mesocarp Fiber for Xylooligosaccharide and Glucose Production" Molecules 23, no. 6: 1310. https://doi.org/10.3390/molecules23061310
APA StyleAhmad, N., Zakaria, M. R., Mohd Yusoff, M. Z., Fujimoto, S., Inoue, H., Ariffin, H., Hassan, M. A., & Shirai, Y. (2018). Subcritical Water-Carbon Dioxide Pretreatment of Oil Palm Mesocarp Fiber for Xylooligosaccharide and Glucose Production. Molecules, 23(6), 1310. https://doi.org/10.3390/molecules23061310