Membrane Fatty Acid Composition and Cell Surface Hydrophobicity of Marine Hydrocarbonoclastic Alcanivorax borkumensis SK2 Grown on Diesel, Biodiesel and Rapeseed Oil as Carbon Sources
Abstract
:1. Introduction
2. Results
2.1. Effect of Growth Substrate on Growth Rates and Cell Surface Properties of A. Borkumensis
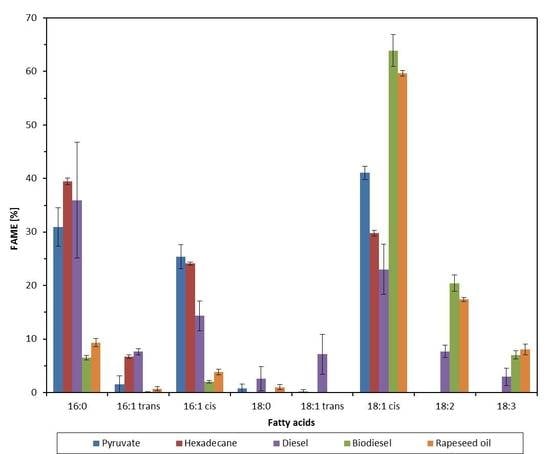
2.2. Effect of Growth Substrate on Membrane Fatty Acid Composition of A. Borkumensis
3. Discussion
4. Materials and Methods
4.1. Strain and Chemicals
4.2. Culture Conditions
4.3. Lipid Extraction, Transesterification, and Fatty Acid Analysis
4.4. Analysis of Fatty Acid Composition by GC-MS
4.5. Analysis of Fatty Acid Composition by GC-FID
4.6. Characterisation of Bacterial Cell Surface Hydrophobicities
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Golyshin, P.N.; Dos Santos, V.; Kaiser, O.; Ferrer, M.; Sabirova, Y.S.; Lunsdorf, H.; Chernikova, T.N.; Golyshina, O.V.; Yakimov, M.M.; Puhler, A.; et al. Genome sequence completed of Alcanivorax borkumensis, a hydrocarbon-degrading bacterium that plays a global role in oil removal from marine systems. J. Biotechnol. 2003, 106, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kalscheuer, R.; Stoveken, T.; Malkus, U.; Reichelt, R.; Golyshin, P.N.; Sabirova, J.S.; Ferrer, M.; Timmis, K.N.; Steinbuchel, A. Analysis of storage livid accumulation in Alcanivorax borkumensis: Evidence for alternative triacylglycerol biosynthesis routes in bacteria. J. Bacteriol. 2007, 189, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Schneiker, S.; dos Santos, V.; Bartels, D.; Bekel, T.; Brecht, M.; Buhrmester, J.; Chernikova, T.N.; Denaro, R.; Ferrer, M.; Gertler, C.; et al. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat. Biotechnol. 2006, 24, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, A.; Baik, S.; Syutsubo, K.; Misawa, N.; Smits, T.H.M.; van Beilen, J.B.; Harayama, S. Cloning and functional analysis of alkB genes in Alcanivorax borkumensis SK2. Environ. Microbiol. 2004, 6, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Denaro, R.; Genovese, M.; Cappello, S.; D’Auria, G.; Chernikova, T.N.; Timmis, K.N.; Golyshin, P.N.; Giluliano, L. Natural microbial diversity in superficial sediments of Milazzo Harbor (Sicily) and community successions during microcosm enrichment with various hydrocarbons. Environ. Microbiol. 2005, 7, 1426–1441. [Google Scholar] [CrossRef] [PubMed]
- Sabirova, J.S.; Ferrer, M.; Regenhardt, D.; Timmis, K.N.; Golyshin, P.N. Proteomic insights into metabolic adaptations in Alcanivorax borkumensis induced by alkane utilization. J. Bacteriol. 2006, 188, 3763–3773. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Syutsubo, K.; Harayama, S. Alcanivorax which prevails in oil-contaminated seawater exhibits broad substrate specificity for alkane degradation. Environ. Microbiol. 2003, 5, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Naether, D.J.; Slawtschew, S.; Stasik, S.; Engel, M.; Olzog, M.; Wick, L.Y.; Timmis, K.N.; Heipieper, H.J. Adaptation of the hydrocarbonoclastic bacterium Alcanivorax borkumensis SK2 to alkanes and toxic organic compounds: A physiological and transcriptomic approach. Appl. Environ. Microbiol. 2013, 79, 4282–4293. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.J.; Loffeld, B.; Keweloh, H.; de Bont, J.A.M. The cis/trans isomerization of unsaturated fatty acids in Pseudomonas putida S12: An indicator for environmental stress due to organic compounds. Chemosphere 1995, 30, 1041–1051. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Golyshin, P.N.; Lang, S.; Moore, E.R.B.; Abraham, W.R.; Lunsdorf, H.; Timmis, K.N. Alcanivorax borkumensis gen. nov. sp. nov. a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Bacteriol. 1998, 48, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Baelum, J.; Borglin, S.; Chakraborty, R.; Fortney, J.L.; Lamendella, R.; Mason, O.U.; Auer, M.; Zemla, M.; Bill, M.; Conrad, M.E.; et al. Deep-sea bacteria enriched by oil and dispersant from the Deepwater Horizon spill. Environ. Microbiol. 2012, 14, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Beazley, M.J.; Martinez, R.J.; Rajan, S.; Powell, J.; Piceno, Y.M.; Tom, L.M.; Andersen, G.L.; Hazen, T.C.; Van Nostrand, J.D.; Zhou, J.Z.; et al. Microbial community analysis of a coastal salt marsh affected by the Deepwater Horizon oil spill. PLoS ONE 2012, 7, e41305. [Google Scholar] [CrossRef]
- Hazen, T.C.; Dubinsky, E.A.; DeSantis, T.Z.; Andersen, G.L.; Piceno, Y.M.; Singh, N.; Jansson, J.K.; Probst, A.; Borglin, S.E.; Fortney, J.L.; et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 2010, 330, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kasai, Y.; Kishira, H.; Sasaki, T.; Syutsubo, K.; Watanabe, K.; Harayama, S. Predominant growth of Alcanivorax strains in oil-contaminated and nutrient-supplemented sea water. Environ. Microbiol. 2002, 4, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kostka, J.E.; Prakash, O.; Overholt, W.A.; Green, S.J.; Freyer, G.; Canion, A.; Delgardio, J.; Norton, N.; Hazen, T.C.; Huettel, M. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl. Environ. Microbiol. 2011, 77, 7962–7974. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.M.; Deng, Y.; Van Nostrand, J.D.; He, Z.L.; Voordeckers, J.; Zhou, A.F.; Lee, Y.J.; Mason, O.U.; Dubinsky, E.A.; Chavarria, K.L.; et al. Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J. 2012, 6, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Mason, O.U.; Hazen, T.C.; Borglin, S.; Chain, P.S.G.; Dubinsky, E.A.; Fortney, J.L.; Han, J.; Holman, H.Y.N.; Hultman, J.; Lamendella, R.; et al. Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. ISME J. 2012, 6, 1715–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azocar, L.; Ciudad, G.; Heipieper, H.J.; Navia, R. Biotechnological processes for biodiesel production using alternative oils. Appl. Microbiol. Biotechnol. 2010, 88, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, D.; Marchut-Mikolajczyk, O.; Strzelecki, B.; Gajewska, M.; Polewczyk, A.; Antczak, T. The effect of tert-butylhydroquinone (TBHQ) on biodiesel bioremediation in soil samples inoculated with bacterial cells. Int. Biodeterior. Biodegrad. 2016, 115, 205–211. [Google Scholar] [CrossRef]
- Mohanty, G.; Mukherji, S. Biodegradation rate of diesel range n-alkanes by bacterial cultures Exiguobacterium aurantiacum and Burkholderia cepacia. Int. Biodeterior. Biodegrad. 2008, 61, 240–250. [Google Scholar] [CrossRef]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: Cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Heipieper, H.J.; de Bont, J.A.M. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of the fatty acid composition of membranes. Appl. Environ. Microbiol. 1994, 60, 4440–4444. [Google Scholar] [PubMed]
- Kabelitz, N.; Santos, P.M.; Heipieper, H.J. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 2003, 220, 223–227. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Neumann, G.; Cornelissen, S.; Meinhardt, F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl. Microbiol. Biotechnol. 2007, 74, 961–973. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.; Wick, L.Y.; Heipieper, H.J. Cell wall adaptations of planktonic and biofilm Rhodococcus erythropolis cells to growth on C5 to C16 n-alkane hydrocarbons. Appl. Microbiol. Biotechnol. 2009, 82, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wick, L.Y.; de Munain, A.R.; Springael, D.; Harms, H. Responses of Mycobacterium sp. LB501T to the low bioavailability of solid anthracene. Appl. Microbiol. Biotechnol. 2002, 58, 378–385. [Google Scholar] [PubMed]
- Van Beilen, J.B.; Funhoff, E.G. Alkane hydroxylases involved in microbial alkane degradation. Appl. Microbiol. Biotechnol. 2007, 74, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Van Beilen, J.B.; Marin, M.M.; Smits, T.H.M.; Rothlisberger, M.; Franchini, A.G.; Witholt, B.; Rojo, F. Characterization of two alkane hydroxylase genes from the marine hydrocarbonoclastic bacterium Alcanivorax borkumensis. Environ. Microbiol. 2004, 6, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, A.; Ellingsen, T.E.; Kotlar, H.K.; Zotchev, S.B.; Throne-Holst, M. Bacterial metabolism of long-chain n-alkanes. Appl. Microbiol. Biotechnol. 2007, 76, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Ron, E.Z.; Rosenberg, E. Biosurfactants and oil bioremediation. Curr. Opin. Biotechnol. 2002, 13, 249–252. [Google Scholar] [CrossRef]
- Atashgahi, S.; Sánchez-Andrea, I.; Heipieper, H.J.; van der Meer, J.R.; Stams, A.J.M.; Smidt, H. Prospects for harnessing biocide resistance for bioremediation and detoxification. Science 2018, 360, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [PubMed]
- Van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J.B. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 1987, 53, 1898–1901. [Google Scholar] [PubMed]
- Rijnaarts, H.H.M.; Norde, W.; Lyklema, J.; Zehnder, A.J.B. The isoelectric point of bacteria as indicator for the presence of cell surface polymers that inhibit adhesion. Colloids Surf. B Biointerfaces 1995, 4, 191–197. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczna, M.; Olzog, M.; Naether, D.J.; Chrzanowski, Ł.; Heipieper, H.J. Membrane Fatty Acid Composition and Cell Surface Hydrophobicity of Marine Hydrocarbonoclastic Alcanivorax borkumensis SK2 Grown on Diesel, Biodiesel and Rapeseed Oil as Carbon Sources. Molecules 2018, 23, 1432. https://doi.org/10.3390/molecules23061432
Konieczna M, Olzog M, Naether DJ, Chrzanowski Ł, Heipieper HJ. Membrane Fatty Acid Composition and Cell Surface Hydrophobicity of Marine Hydrocarbonoclastic Alcanivorax borkumensis SK2 Grown on Diesel, Biodiesel and Rapeseed Oil as Carbon Sources. Molecules. 2018; 23(6):1432. https://doi.org/10.3390/molecules23061432
Chicago/Turabian StyleKonieczna, Maria, Martin Olzog, Daniela J. Naether, Łukasz Chrzanowski, and Hermann J. Heipieper. 2018. "Membrane Fatty Acid Composition and Cell Surface Hydrophobicity of Marine Hydrocarbonoclastic Alcanivorax borkumensis SK2 Grown on Diesel, Biodiesel and Rapeseed Oil as Carbon Sources" Molecules 23, no. 6: 1432. https://doi.org/10.3390/molecules23061432
APA StyleKonieczna, M., Olzog, M., Naether, D. J., Chrzanowski, Ł., & Heipieper, H. J. (2018). Membrane Fatty Acid Composition and Cell Surface Hydrophobicity of Marine Hydrocarbonoclastic Alcanivorax borkumensis SK2 Grown on Diesel, Biodiesel and Rapeseed Oil as Carbon Sources. Molecules, 23(6), 1432. https://doi.org/10.3390/molecules23061432