Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives
Abstract
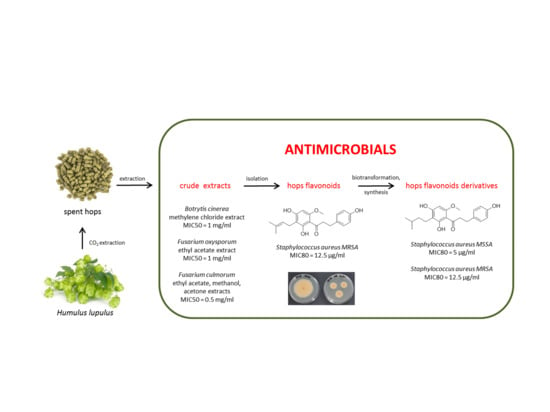
:1. Introduction
2. Results
2.1. Production of Type I and Type II Extracts
2.2. Antibacterial Activity
2.3. Antifungal Activity
3. Discussion
3.1. Antibacterial Activity
3.2. Antifungal Activity
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Extracts
4.3. Flavonoids
4.4. Microorganisms
4.5. Determination of Antibacterial Activity
4.6. Determination of Antifungal Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases on fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Da Cruz Cabral, L.; Virginia Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V. Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 1–414. ISBN 9781498741316. [Google Scholar]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livestock Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Tănase, C.; Coşarcă, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial activities of beech bark (Fagus sylvatica L.) polyphenolic extract. Environ. Eng. Manag. J. 2018, 17, 877–884. [Google Scholar] [CrossRef]
- Simpson, W.J.; Smith, A.R. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharm. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.R.; Frank, N.; Bartsch, H.; et al. Cancer Chemopreventive Activity of Xanthohumol, a Natural Product Derived from Hop. Mol. Cancer Ther. 2002, 1, 959–969. [Google Scholar] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; van Breemen, R.B. Identification of a potent phytoestrogen in hops (Humulus lupulus L.), and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.J.; Barsoumian, R. Antimicrobial activity of hop resins. J. Food Prot. 1994, 57, 59–61. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Virani, S.; Zavro, M.; Haas, G.J. Inhibition of Streptococcus mutans and other oral streptococci by hop (Humulus lupulus L.) constituents. Econ. Bot. 2003, 57, 118–125. [Google Scholar] [CrossRef]
- Shen, C.; Geornaras, I.; Kendall, P.A.; Sofos, J.N. Control of Listeria monocytogenes on frankfurters by dipping in hops beta acids solutions. J. Food Prot. 2009, 72, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.R.; Haas, G.J.; Matthews, P.D.; Smith, R.J.; Buhr, R.J.; Dale, N.M.; Wise, M.G. Antimicrobial activity of lupulone against Clostridium perfringens in the chicken intestinal tract jejunum and caecum. J. Antimicrob. Chemother. 2008, 61, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Teuber, M.; Schmalreck, A.F. Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone and humulinic acid. Arch. Microbiol. 1973, 94, 159–171. [Google Scholar] [CrossRef]
- Ohsugi, M.; Basnet, P.; Kadota, S.; Ishii, E.; Tamura, T.; Okumura, Y.; Namba, T. Antibacterial activity of traditional medicines and an active constituent lupulone from Humulus lupulus against Helicobacter. pylori. J. Trad. Med. 1997, 14, 186–191. [Google Scholar]
- Shapouri, R.; Rahnema, M. Evaluation of antimicrobial effect of hops extracts on intramacrophages Brucella abortus and B. melitensis. Jundishapur J. Microbiol. 2011, 4, 51–58. [Google Scholar]
- Schmalreck, A.F.; Teuber, M.; Reininger, W.; Hartl, A. Structural features determining the antibiotic potencies of natural and synthetic hop bitter resins, their precursors and derivatives. Can. J. Microbiol. 1975, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, S.; Sato, Y. A new flavanone with antifungal activity isolated from hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Różalski, M.; Micota, B.; Sadowska, B.; Stochmal, A.; Jędrejek, D.; Więckowska-Szakiel, M.; Różalska, B. Antiadherent and antibiofilm activity of Humulus lupulus L. derived products: New pharmacological properties. BioMed. Res. Int. 2013, 2013, 101089. [Google Scholar] [CrossRef] [PubMed]
- Rój, E.; Tadić, V.M.; Misić, D.; Žižović, I.; Arsić, I.; Dobrzyńska-Inger, A.; Kostrzewa, D. Supercritical carbon dioxide hops extracts with antimicrobial properties. Open Chem. 2015, 13, 157–1171. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Alcaráz, L.E.; Blanco, S.E.; Puig, O.N.; Tomás, F.; Ferretti, F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Popłoński, J.; Tronina, T.; Sordon, S.; Huszcza, E. Selective hydrogenation of xanthohumol to α,β-dihydroxanthohumol. Przem. Chem. 2014, 93, 1916–1918. [Google Scholar]
- Tronina, T.; Bartmańska, A.; Filip-Psurska, B.; Wietrzyk, J.; Popłoński, J.; Huszcza, E. Fungal metabolites of xanthohumol with potent antiproliferative activity on human cancer cell lines in vitro. Bioorg. Med. Chem. 2013, 21, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.D.; Shovan, L.R.; Hjeljord, L.G.; Aam, B.B.; Eijsink, V.G.H.; Sørlie, M.; Tronsmo, A. Inhibition of fungal plant pathogens by synergistic action of chito-oligosaccharides and commercially available fungicides. PLoS ONE 2014, 9, E93192. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackowski, J.; Hurej, M.; Rój, E.; Popłoński, J.; Kośny, L.; Huszcza, E. Antifeedant activity of xanthohumol and supercritical carbon dioxide extract of spent hops against stored product pests. Bull. Entomol. Res. 2015, 105, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Strugała, P.; Popłoński, J.; Włoch, A.; Sordon, S.; Bartmańska, A.; Huszcza, E. The influence of glycosylation of natural and synthetic prenylated flavonoids on binding to human serum albumin and inhibition of cyclooxygenases COX-1 and COX-2. Molecules 2017, 22, 1230. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and antiproliferative activity of glycosides obtained by biotransformation of xanthohumol. Bioorg. Med. Chem. Lett. 2013, 23, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Popłoński, J.; Turlej, E.; Sordon, S.; Tronina, T.; Bartmańska, A.; Wietrzyk, J.; Huszcza, E. Synthesis and antiproliferative activity of hops minor prenylflavonoids and new insights on prenyl group cyclization. Molecules 2018, 23, 776. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Huszcza, E. Transformation of xanthohumol by Aspergillus ochraceus. J. Basic. Microbiol. 2013, 53, 1–6. [Google Scholar]
- Artmańska, A.; Huszcza, E.; Tronina, T. Transformation of isoxanthohumol by fungi. J. Mol. Catal. B. Enzym. 2009, 61, 221–224. [Google Scholar] [CrossRef]
- Anioł, M.; Szymańska, K.; Żołnierczyk, A. An efficient synthesis of the phytoestrogen 8-prenylnaringenin from isoxanthohumol with magnesium iodide etherate. Tetrahedron 2008, 64, 9544–9547. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Huszcza, E. Transformation of 8-prenylnaringenin by Absidia coerulea and Beauveria bassiana. Bioorg. Med. Chem. Lett. 2012, 22, 6451–6453. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–26 are available from the authors. |

| Extracts Type I | Xanthohumol [µg/mg] a | Extracts Type II | Xanthohumol [ng/mg] a |
|---|---|---|---|
| methylene chloride | 51.15 ± 0.14 | methylene chloride | ˂0.5 b |
| ethyl acetate | 90.95 ± 0.28 | ethyl acetate | 397 ± 15.18 |
| acetone | 99.70 ± 0.31 | acetone | 278 ± 17.95 |
| methanol | 31.65 ± 0.60 | methanol | 1.5 ± 0.91 |
| Microorganism | MIC80 [µg/mL] | ||||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 14 | 17 | 20a, b | 26 | Ampicillin | |
| S. aureus ATCC19095 (MSSA) | 12.5 | 5 | >50 | 50 | 12.5 | 12.5 | 2.5 |
| S. aureus ATCC43300 (MRSA) | 12.5 | 12.5 | >50 | 50 | 12.5 | 12.5 | ˃5 |
| S. aureus ATCC29213 (MSSA) | 12.5 | 12.5 | 50 | 25 | 12.5 | 12.5 | ˃5 |
| S. epidermidis 4s (MSSE) | 12.5 | >50 | 50 | 50 | 25 | 12.5 | 2.5 |
| S. epidermidis 91M (MRSE) | 25 | >50 | >50 | 50 | 25 | 25 | ˃5 |
| S. typhimurium PCM2565 | na | na | na | na | na | 50 | 5 |
| L. monocytogenes ATCC7644 | na | na | na | na | na | na | 2.5 |
| Microorganism | Growth Inhibition [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| Methylene Chloride | Ethyl Ccetate | Acetone | Methanol | |||||
| Extract I | Extract II | Extract I | Extract II | Extract I | Extract II | Extract I | Extract II | |
| F. culmorum AM10 | 37.5 ± 0.79 | 34.2 ± 0,40 | 55.9 ± 0.79 | 41.4 ± 0.39 | 54.6 ± 0.39 | 45.2 ± 0.79 | 53.1 ± 0.40 | 41.4 ± 1.04 |
| F. equiseti AM15 | 35.3 ± 1.50 | 20.5 ± 2.04 | 39.7 ± 1.50 | 16.5 ± 1.13 | 44.2 ± 0.57 | 16.9 ± 1.68 | 41.2 ± 0.98 | 32.5 ± 0.98 |
| F. semitectum AM20 | 43.2 ± 0.5 | 15.2 ± 1.29 | 51.3 ± 0.98 | 16.2 ± 0.98 | 54.1 ± 0.85 | 24.6 ± 0.98 | 47.3 ± 2.59 | 31.6 ± 1.76 |
| F. oxysporum AM21 | 31.6 ± 2.68 | 6.1 ± 0.45 | 50.0 ± 0.45 | 7.8 ± 0.77 | 39.5 ± 0.45 | 5.1 ± 0.77 | 36.8 ± 0.45 | 4.7 ± 1.18 |
| B. cinerea AM235 | 50.9 ± 1.17 | 25.4 ± 0.88 | 30.9 ± 1.17 | 5.3 ± 0.76 | 36.4 ± 0.44 | 12.1 ± 0.44 | 22.4 ± 1.93 | 21.4 ± 1.59 |
| P. purpurogenum AM80 | 14.3 ± 1.95 | 0.0 ± 0.98 | 26.2 ± 1.95 | 9.9 ± 0.97 | 23.8 ± 1.69 | 9.1 ± 0.97 | 26.2 ± 0.98 | 1.0 ± 0.98 |
| M. hiemalis AM450 | 39.3 ± 0.6 | 0.0 ± 1.00 | 28.9 ± 1.00 | 0.0 ± 0.50 | 30.0 ± 1,72 | 0.0 ± 0.50 | 30.4 ± 1,79 | 0.0 ± 1.00 |
| Antifungals | MIC50 [mg/mL] | |||
|---|---|---|---|---|
| F. culmorum AM10 | F. semitectum AM20 | F. oxysporum AM21 | B. cinerea AM235 | |
| methylene chloride extract I | ˃1 | ˃1 | ˃1 | 1 |
| ethyl acetate extract I | 0.5 | 1 | 1 | ˃1 |
| acetone extract I | 0.5 | 1 | ˃1 | ˃1 |
| methanol extract I | 0.5 | ˃1 | ˃1 | ˃1 |
| xanthohumol | 0.015 | 0.030 | 0.100 | ˃0.200 |
| amphotericin B | 0.005 | 0.005 | 0.005 | 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. https://doi.org/10.3390/molecules23082059
Bartmańska A, Wałecka-Zacharska E, Tronina T, Popłoński J, Sordon S, Brzezowska E, Bania J, Huszcza E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules. 2018; 23(8):2059. https://doi.org/10.3390/molecules23082059
Chicago/Turabian StyleBartmańska, Agnieszka, Ewa Wałecka-Zacharska, Tomasz Tronina, Jarosław Popłoński, Sandra Sordon, Ewa Brzezowska, Jacek Bania, and Ewa Huszcza. 2018. "Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives" Molecules 23, no. 8: 2059. https://doi.org/10.3390/molecules23082059
APA StyleBartmańska, A., Wałecka-Zacharska, E., Tronina, T., Popłoński, J., Sordon, S., Brzezowska, E., Bania, J., & Huszcza, E. (2018). Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules, 23(8), 2059. https://doi.org/10.3390/molecules23082059