Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway
Abstract
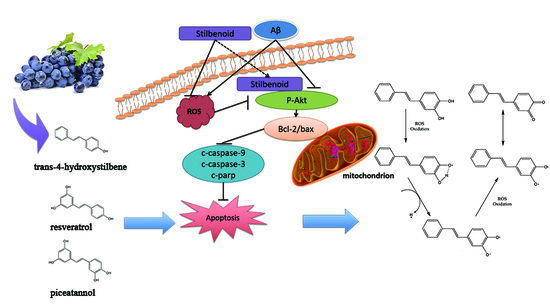
:1. Introduction
2. Results
2.1. Free Radical Scavenging Activity of Stilbenoids on DPPH• and •OH
2.2. Effects of Stilbenoids on Intracellular ROS in Aβ25-35-Induced Neurons
2.3. Effects of Stilbenoids on Cell Viability in Aβ25-35-Induced Neurons
2.4. Effects of Stilbenoids on Akt Phosphorylation in Aβ25-35-Induced Neurons
2.5. Effects of Stilbenoids on Bcl-2 Family Proteins in Aβ25-35-Induced Neurons
2.6. Effects of Stilbenoids on Multiple Caspases in Aβ25-35-Induced Neurons
2.7. Molecular Docking of Stilbenoids with Akt Protein
3. Discussion
3.1. Stilbenoids Suppress Aβ25-35-Induced Neurotoxicity by Reducing ROS via PI3k/Akt Signalling Pathway in Neurons
3.2. The Relationship between Antioxidant, Neuroprotective Activity, and Chemical Structure of Stilbenoids
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Cell Culture and Peptide Preparation
4.3. In Vitro Antioxidant Activity
4.4. Intracellular Reactive Oxygen Species (ROS) Measurement
4.5. Cell Viability
4.6. Western Blotting
4.7. Molecular Docking
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mathiyazahan, D.B.; Thenmozhi, A.J.; Manivasagam, T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Lee, C.L.; Lin, P.Y.; Hsu, Y.W.; Pan, T.M. Monascus-fermented monascin and ankaflavin improve the memory and learning ability in amyloid beta-protein intracerebroventricular-infused rat via the suppression of Alzheimer’s disease risk factors. J. Funct. Foods 2015, 18, 387–399. [Google Scholar] [CrossRef]
- Lu, J.X.; Qiang, W.; Yau, W.M.; Schwieters, C.D.; Meredith, S.C.; Tycko, R. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 2011, 154, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Benilova, I.; Karran, E.; de Strooper, B. The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Reed, T.; Newman, S.F.; Sultana, R. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Zhao, B.; Ratka, A. Oxidative Stress and β-Amyloid Protein in Alzheimer’s Disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.G.; Oliveira, J.M.; Carvalho, L.P.; da Cruz e Silva, O.A.B. A beta Influences Cytoskeletal Signaling Cascades with Consequences to Alzheimer’s Disease. Mol. Neurobiol. 2015, 52, 1391–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Raina, A.K.; Lee, H.G.; Casadesus, G.; Smith, M.A.; Perry, G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004, 1000, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Akca, H.; Demiray, A.; Tokgun, O.; Yokota, J. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells. Lung Cancer 2011, 73, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Wang, X.M.; Ko, H.; Kwon, H.C.; Cha, J.W.; Yang, H.O. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/BclXL-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2011, 672, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, X.; Zhao, S.; Wei, C.; Yin, Y.; Liu, T.; Jiang, S.; Xie, J.; Wan, X.; Mao, M.; et al. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem. Int. 2013, 63, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.J.; Wei, Q.Y.; Fang, J.G.; Yang, L.; Liu, Z.L.; Wyche, J.H.; Han, Z. The 3,4-dihydroxyl groups are important for trans-resveratrol analogs to exhibit enhanced antioxidant and apoptotic activities. Anticancer Res. 2004, 24, 999–1002. [Google Scholar] [PubMed]
- Pawlus, A.D.; Sahli, R.; Bisson, J.; Rivière, C.; Delaunay, J.C.; Richard, T.; Gomès, E.; Bordenave, L.; Waffotéguo, P.; Mérillon, J.M. Stilbenoid Profiles of Canes from Vitis and Muscadinia Species. J. Agric. Food Chem. 2012, 61, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, T.; Thudium, D.; Gronbaek, M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Papastamoulis, Y.; Fortin, P.Y.; Delchier, N.; Andriamanarivo, S.; Waffoteguo, P.; Kapche, G.D.; Amiraguebalia, H.; Delaunay, J.C.; Mérillon, J.M. New stilbene dimers against amyloid fibril formation. Bioorg. Med. Chem. Lett. 2010, 20, 3441–3443. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.W.; Liang, N.; Zhu, D.X.; Gao, Q.; Peng, L.; Dong, H.M.; Yue, Q.W.; Liu, H.L.; Bao, L.H.; Zhang, J.; et al. Resveratrol Inhibits beta-Amyloid-Induced Neuronal Apoptosis through Regulation of SIRT1-ROCK1 Signaling Pathway. PLoS ONE 2013, 8, e59888. [Google Scholar]
- Fu, Z.; Wei, Y.J.; Li, J.M. Effects of piceatannol and pterostilbene against β-amyloid-induced apoptosis on the PI3K/Akt/Bad signaling pathway in PC12 cells. Food Funct. 2016, 7, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Jager, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, J.E.; Kang, N.J.; Lee, K.W.; Lee, H.J. Piceatannol attenuates 4-hydroxynonenal-induced apoptosis of PC12 cells by blocking activation of c-Jun N-terminal kinase. Ann. N. Y. Acad. Sci. 2009, 1171, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, K.W.; Kim, M.S.; Lee, H.J. Piceatannol attenuates hydrogen-peroxide- and peroxynitrite-induced apoptosis of PC12 cells by blocking down-regulation of Bcl-XL and activation of JNK. J. Nutr. Biochem. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- OuYang, F.; Wang, G.; Guo, W.; Zhang, Y.; Xiang, W.; Zhao, M. AKT signalling and mitochondrial pathways are involved in mushroom polysaccharide-induced apoptosis and G1 or S phase arrest in human hepatoma cells. Food Chem. 2013, 138, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Childs, A.C.; Phaneuf, S.L.; Dirks, A.J.; Phillips, T.; Leeuwenburgh, C. Doxorubicin treatment in vivo causes cytochrome c release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002, 62, 4592–4598. [Google Scholar] [PubMed]
- Maira, S.M.; Galetic, I.; Brazil, D.P.; Kaech, S.; Ingley, E.; Thelen, M.; Hemmings, B.A. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science 2001, 294, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F. Akt-interacting proteins: Attractive opposites. Focus on “Carboxy-terminal modulator protein induces Akt phosphorylation and activation, thereby enhancing antiapoptotic, glycogen synthetic, and glucose uptake pathways”. Am. J. Physiol. Cell Physiol. 2007, 293, C1768–C1770. [Google Scholar] [CrossRef] [PubMed]
- Parcellier, A.; Tintignac, L.A.; Zhuravleva, E.; Dummler, B.; Brazil, D.P.; Hynx, D.; Cron, P.; Schenk, S.; Olivieri, V.; Hemmings, B.A. The Carboxy-Terminal Modulator Protein (CTMP) regulates mitochondrial dynamics. PLoS ONE 2009, 4, e5471. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhen, Y.F.; Pu-Bu-Ci-Ren; Song, L.G.; Kong, W.N.; Shao, T.M.; Li, X.; Chai, X.Q. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav. Brain Res. 2013, 244, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Yuan, C.; He, J.; Tan, T.P.; Wu, S.J.; Fu, H.Y.; Liu, J.; Yu, S.; Chen, Y.D.; Le, Q.F.; et al. Resveratrol protects PC12 cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a pathway. Cell. Mol. Neurobiol. 2015, 35, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Bournival, J.; Zottig, X.; Martinoli, M.G. Resveratrol protects DAergic PC12 cells from high glucose-induced oxidative stress and apoptosis: Effect on p53 and GRP75 localization. Neurotox. Res. 2014, 25, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Parcellier, A.; Tintignac, L.A.; Zhuravleva, E.; Cron, P.; Schenk, S.; Bozulic, L.; Hemmings, B.A. Carboxy-Terminal Modulator Protein (CTMP) is a mitochondrial protein that sensitizes cells to apoptosis. Cell. Signal. 2009, 21, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.F.; Lin, Z.X.; Mao, Q.Q.; Chen, J.N.; Su, Z.R.; Lai, X.P.; Ip, P.S. Isorhynchophylline Protects PC12 Cells Against Beta-Amyloid-Induced Apoptosis via PI3K/Akt Signaling Pathway. Evid.-Based Complement. Altern. Med. 2014, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.F.; Lin, H.C.; Huang, X.W.; Huang, H.Y.; Wu, C.P.; Kao, M.C. An ethanol extract of Poria cocos inhibits the proliferation of non-small cell lung cancer A549 cells via the mitochondria-mediated caspase activation pathway. J. Funct. Foods 2016, 23, 614–627. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.; Ruberto, G. Kinetic solvent effects on phenolic antioxidants determined by spectrophotometric measurements. J. Agric. Food Chem. 2001, 49, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Fang, J.G.; Chen, W.F.; Zhou, B.; Yang, L.; Liu, Z.L. Structure-activity relationship studies of resveratrol and its analogues by the reaction kinetics of low density lipoprotein peroxidation. Bioorg. Chem. 2006, 34, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Wei, Q.Y.; Cai, Y.J.; Fang, J.G.; Zhou, B.; Yang, L.; Liu, Z.L. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: Mechanism and structure-activity relationship. Free Radic. Biol. Med. 2006, 41, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Nagakawa, M.; Nakanishi, I.; Ohkubo, K.; Imai, K.; Urano, S.; Fukuzumi, S.; Ozawa, T.; Ikota, N.; Mochizuki, M.; et al. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu(II). Bioorg. Med. Chem. 2006, 14, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Millucci, L.; Raggiaschi, R.; Franceschini, D.; Terstappen, G.; Santucci, A. Rapid aggregation and assembly in aqueous solution of Aβ (25–35) peptide. J. Biosci. 2009, 34, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Chen, C. Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neuroscience 2011, 178, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.J.; Zhao, S.R.; Li, J.M.; Xue, B. Stilbene profiles in different tissues of Vitis vinifera L. cv. Cabernet Sauvignon and a comparison of their antioxidant activity. Aust. J. Grape Wine Res. 2016, 22, 226–231. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Stilbenoids | Binding Energy (Kcal/mol) | Ki (μM) | Final Intermolecular Energy (Kcal/mol) | VDW + H Bond + Desolvation Energy (Kcal/mol) | H Bonds |
|---|---|---|---|---|---|
| trans-4-hydroxystilbene | −6.31 ± 0.33 | 23.78 | −7.2 | −7.1 | THS:O1H1-GLU432:O |
| ASN231:ND2-THS:O1 | |||||
| resveratrol | −6.69 ± 0.68 | 12.54 | −8.18 | −7.82 | RES:O1-LEU213:O |
| RES:O2-TYR229:O | |||||
| LYS284:NZ-RES:O2 | |||||
| TYR175:HN-RES:O3 | |||||
| piceatannol | −6.73 ± 0.51 | 11.75 | −8.52 | −8.28 | PIC:HAD-ILE447:O |
| PIC:H1-ILE447:O | |||||
| PIC:H2-PRO451:O | |||||
| PIC:H3-PHE150:O | |||||
| PIC:H3-MET147:O |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules 2018, 23, 2328. https://doi.org/10.3390/molecules23092328
Wen H, Fu Z, Wei Y, Zhang X, Ma L, Gu L, Li J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules. 2018; 23(9):2328. https://doi.org/10.3390/molecules23092328
Chicago/Turabian StyleWen, Haichao, Zheng Fu, Yangji Wei, Xiaoxu Zhang, Liyan Ma, Liwei Gu, and Jingming Li. 2018. "Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway" Molecules 23, no. 9: 2328. https://doi.org/10.3390/molecules23092328
APA StyleWen, H., Fu, Z., Wei, Y., Zhang, X., Ma, L., Gu, L., & Li, J. (2018). Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules, 23(9), 2328. https://doi.org/10.3390/molecules23092328