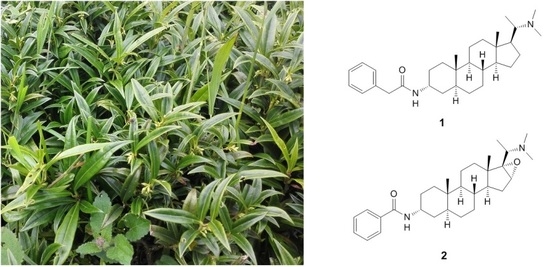
Two New Cytotoxic Steroidal Alkaloids from Sarcococca Hookeriana
Abstract
:1. Introduction
2. Results and Discussion
2.1. Elucidation of the Chemical Structure of Compounds
2.2. Results of the Cytotoxicity Test
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Compound 1
3.3.2. Compound 2
3.4. Single Crystal X-Ray Data of Compound 4
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Editorial Committee of Flora of China. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 2004; Volume 45, pp. 41–56. [Google Scholar]
- Yu, S.S.; Zou, Z.M.; Zen, J.; Yu, D.Q.; Cong, P.Z. Four new steroidal alkaloids from the roots of Sarcococca vagans. Chin. Chem. Lett. 1997, 8, 511–514. [Google Scholar]
- He, K.; Du, J. Two New steroidal alkaloids from the roots of Sarcococca ruscifolia. J. Asian Nat. Prod. Res. 2010, 12, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, M.N.; Gilani, A.H. Studies on cardio-suppressant, vasodilator and tracheal relaxant effects of Sarcococca saligna. Arch. Pharm. Res. 2006, 29, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Lenta, B.N.; Fokou, P.A.; Sewald, N. Terpenoid alkaloids of the Buxaceae family with potential biological importance. Nat. Prod. Rep. 2008, 25, 612–630. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Feroz, F.; Naeem, I.; Haq, Z.; Nawaz, S.A.; Khan, N.; Khan, M.R.; Choudhary, M.I. New pregnane-type steroidal alkaloids from Sarcococca saligna and their cholinesterase inhibitory activity. Steroids 2004, 69, 735–741. [Google Scholar] [CrossRef]
- Rahman, A.; Haq, Z.; Fareeda, F.; Khalid, A.; Nawaz, S.A.; Khan, M.R.; Choudhary, M. New cholinesterase-inhibiting steroidal alkaloids from Sarcococca saligna. Helv. Chim. Acta 2004, 87, 439–448. [Google Scholar] [CrossRef]
- Yan, Y.X.; Sun, Y.; Chen, J.C.; Wang, Y.Y.; Li, Y.; Qiu, M.H. Cytotoxic steroids from Sarcococca saligna. Planta Med. 2011, 77, 1725–1729. [Google Scholar] [CrossRef]
- Rahman, A.; Anjum, S.; Farooq, A.; Khan, M.R.; Choudhary, M. Two new pregnane-type steroidal alkaloids from Sarcococca saligna. Phytochemistry 1997, 46, 771–775. [Google Scholar] [CrossRef]
- Devkota, K.P.; Choudhary, M.I.; Ranjit, R.; Samreen; Sewald, N. Structure activity relationship studies on antileishmanial steroidal alkaloids from Sarcococca hookeriana. Nat. Prod. Res. 2007, 21, 292–297. [Google Scholar] [CrossRef]
- Ullah Jan, N.; Ali, A.; Ahmad, B.; Iqbal, N.; Adhikari, A.; Inayat Ur, R.; Musharraf, S.G. Evaluation of antidiabetic potential of steroidal alkaloid of Sarcococca saligna. Biomed. Pharm. 2018, 100, 461–466. [Google Scholar] [CrossRef]
- Zhang, P.Z.; Wang, F.; Yang, L.J.; Zhang, G.L. Pregnane alkaloids from Sarcococca hookeriana var. digyna. Fitoterapia 2013, 89, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Devkota, K.P.; Nawaz, S.A.; Shaheen, F.; Rahman, A. Cholinesterase-inhibiting new steroidal alkaloids from Sarcococca hookeriana of nepalese origin. Helv. Chim. Acta 2004, 87, 1099–1108. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Devkota, K.P.; Nawaz, S.A.; Ranjit, R.; Rahman, A. Cholinesterase inhibitory pregnane-type steroidal alkaloids from Sarcococca hookeriana. Steroids 2005, 70, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Lenta, B.N.; Choudhary, M.I.; Naz, Q.; Fekam, F.B.; Rosenthal, P.J. Cholinesterase inhibiting and antiplasmodial steroidal alkaloids from Sarcococca hookeriana. Chem. Pharm. Bull. 2007, 55, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Lenta, B.N.; Wansi, J.D.; Choudhary, M.I.; Kisangau, D.P.; Naz, Q.; Samreen; Sewald, N. Bioactive 5α-pregnane-type steroidal alkaloids from Sarcococca hookeriana. J. Nat. Prod. 2008, 71, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Wansi, J.D.; Lenta, B.N.; Khan, S.; Choudhary, M.I.; Sewald, N. Bioactive steroidal alkaloids from Sarcococca hookeriana. Planta Med. 2010, 76, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, J.X.; Zou, J.; Wu, J.C.; Huo, S.J.; Du, J. Three new cytotoxic steroidal alkaloids from Sarcococca hookeriana. Molecules 2018, 23, 1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shao, L.; Shi, Z.; Zhang, Y.; Du, J.; Cheng, K.J. Pregnane alkaloids from Sarcococca ruscifolia and their cytotoxic activity. Phytochem. Lett. 2015, 14, 31–34. [Google Scholar] [CrossRef]
- Rahman, A.; Anjum, S.; Farooq, A.; Khan, M.R.; Parveen, Z.; Choudhary, M.I. Antibacterial steroidal alkaloids from Sarcococca saligna. J. Nat. Prod. 1998, 61, 202–206. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta. Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, M.; Li, K.J.; Sun, S.; Xing, G.Q.; Gaoe, R.; Leia, Z.F. Anti-tumor and anti-metastasis activities of honey bee larvae powder by suppressing the expression of EZH2. Biomed. Pharma. 2018, 105, 690–696. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |

 ), HMBC (H→C), and ROESY (
), HMBC (H→C), and ROESY (  ) correlations of compound 1.
) correlations of compound 1.
 ), HMBC (H→C), and ROESY (
), HMBC (H→C), and ROESY (  ) correlations of compound 2.
) correlations of compound 2.

| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.45 (m), 0.65 (m) | 33.2 (t) | 1.72 (m), 1.10 (m) | 37.2 (t) |
| 2 | 1.63 (m), 1.52 (m) | 25.9 (t) | 1.93 (d, J = 14.8), 1.38 (m) | 28.8 (t) |
| 3 | 4.06 (m) | 44.8 (d) | 3.96 (m) | 49.3 (d) |
| 4 | 1.45 (m), 1.28 (m) | 32.7 (t) | 1.70 (m), 1.24 (m) | 35.4 (t) |
| 5 | 0.79 (m) | 41.0 (d) | 1.24 (m) | 45.4 (d) |
| 6 | 1.12 (m) | 28.5 (t) | 1.32 (m), 1.24 (m) | 28.4 (t) |
| 7 | 1.64 (m), 0.77 (m) | 32.1 (t) | 1.62 (m), 0.95 (m) | 31.6 (t) |
| 8 | 1.31 (m) | 35.4 (d) | 1.62 (m) | 33.8 (d) |
| 9 | 0.46 (d, J = 11.8, 3.9) | 54.7 (d) | 0.73 (m) | 54.5 (d) |
| 10 | 36.0 (s) | 35.6 (s) | ||
| 11 | 1.42 (m), 1.18 (m) | 20.8 (t) | 1.62 (m), 1.29 (m) | 20.8 (t) |
| 12 | 1.87 (m), 1.08 (m) | 39.8 (t) | 1.62 (m), 1.47 (m) | 32.8 (t) |
| 13 | 41.7 (s) | 42.2 (s) | ||
| 14 | 1.01 (m) | 56.8 (d) | 1.24 (m) | 45.1 (d) |
| 15 | 1.58 (m), 1.02 (m) | 24.0 (t) | 1.84 (dd, J = 12.0, 4.8), 1.20 (m) | 27.2 (t) |
| 16 | 1.85 (m), 1.44 (m) | 27.7 (t) | 3.55 (s) | 60.9 (d) |
| 17 | 1.36 (d, J = 9.9) | 54.9 (d) | 73.6 (s) | |
| 18 | 0.63 (s) | 12.4 (q) | 0.87 (s) | 15.8 (q) |
| 19 | 0.74 (s) | 11.5 (q) | 0.83 (s) | 12.2 (q) |
| 20 | 2.41 (dq, J = 10.2, 6.4) | 61.2 (d) | 2.84 (q, J = 6.6) | 55.7 (d) |
| 21 | 0.87 (s) | 10.0 (q) | 1.14 (s) | 13.6 (q) |
| N(Me)2 | 2.17 (s) | 39.9 (q) | 2.24 (s) | 43.1 (q) |
| 1′ | 170.1 (s) | 166.7 (s) | ||
| 2′ | 3.57 (dt, J = 6.8, 1.2) | 44.2 (t) | 135.0 (s) | |
| 3′ | 135.5 (s) | 7.74 (dt, J = 6.8, 1.2) | 126.8 (d) | |
| 4′ | 7.28 (m) | 129.4 (d) | 7.41 (m) | 128.5 (d) |
| 5 | 7.37 (m) | 129.1 (d) | 7.47 (m) | 131.2 (d) |
| 6′ | 7.33 (m) | 127.4 (d) | 7.41 (m) | 128.5 (d) |
| 7′ | 7.37 (m) | 129.1 (d) | 7.47 (m) | 126.8 (d) |
| 8′ | 7.28 (m) | 129.4 (d) | ||
| NH | 5.66 (d, J = 8.0) | 5.98 (d, J = 8.0) |
| Compounds | IC50 (μM) a (n = 3) | ||||
|---|---|---|---|---|---|
| SW480 | SMMC-7721 | PC3 | MCF-7 | K562 | |
| 1 | 10.97 ± 1.36 | 41.31 ± 3.02 | 32.97 ± 3.78 | 37.30 ± 0.99 | 11.86 ± 0.82 |
| 2 | 5.97 ± 0.13 | 16.19 ± 0.56 | 11.57 ± 0.86 | 19.44 ± 1.70 | 7.95 ± 0.02 |
| 3 | 5.77 ± 0.29 | 10.84 ± 1.19 | 11.79 ± 2.96 | 44.97 ± 4.73 | 6.29 ± 0.53 |
| 4 | 45.92 ± 1.56 | 71.13 ± 5.37 | >100 | 28.92 ± 1.22 | 85.48 ± 6.77 |
| DDP b | 4.71 ± 0.20 | 4.03 ± 0.62 | 6.50 ± 0.44 | 6.86 ± 0.42 | 5.49 ± 0.83 |
| 5-FU c | 7.65 ± 0.26 | 7.86 ± 0.38 | 8.18 ± 0.73 | 6.74 ± 0.89 | 4.78 ± 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, S.; Wu, J.; He, X.; Pan, L.; Du, J. Two New Cytotoxic Steroidal Alkaloids from Sarcococca Hookeriana. Molecules 2019, 24, 11. https://doi.org/10.3390/molecules24010011
Huo S, Wu J, He X, Pan L, Du J. Two New Cytotoxic Steroidal Alkaloids from Sarcococca Hookeriana. Molecules. 2019; 24(1):11. https://doi.org/10.3390/molecules24010011
Chicago/Turabian StyleHuo, Shaojie, Jichun Wu, Xicheng He, Lutai Pan, and Jiang Du. 2019. "Two New Cytotoxic Steroidal Alkaloids from Sarcococca Hookeriana" Molecules 24, no. 1: 11. https://doi.org/10.3390/molecules24010011
APA StyleHuo, S., Wu, J., He, X., Pan, L., & Du, J. (2019). Two New Cytotoxic Steroidal Alkaloids from Sarcococca Hookeriana. Molecules, 24(1), 11. https://doi.org/10.3390/molecules24010011