Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies
Abstract
:1. Introduction
2. Results and Discussion
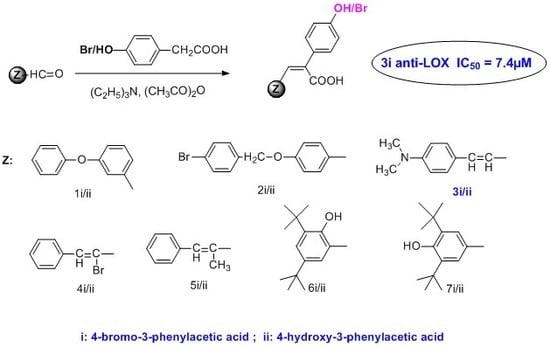
2.1. Chemistry
2.2. Physicochemical Studies
2.3. Biological Evaluation
2.4. Computational Studies—Docking Simulation Soybean Lipoxygenase
Molecular Modeling of the Synthesized Derivatives in Soybean LOX
3. Experimental Section
3.1. Materials and Instruments
3.2. Chemistry General Procedures
3.3. Physicochemical Studies
3.3.1. Determination of RM Values
3.3.2. In Silico Determination of Lipophilicity as Clog P
3.4. Biological In Vitro Assays
3.4.1. Determination of the Reducing Activity of the Stable Radical 1,1-Diphenyl-picrylhydrazyl (DPPH)
3.4.2. Competition of the Tested Compounds with DMSO for Hydroxyl Radicals
3.4.3. Non Enzymatic Assay of Superoxide Radical Scavenging Activity
3.4.4. ABTS+•—Decolorization Assay for Antioxidant Activity
3.4.5. Inhibition of Linoleic Acid Peroxidation
3.4.6. Soybean Lipoxygenase Inhibition Study In Vitro
3.5. Computational Methods—Molecular Docking Studies on Soybean Lipoxygenase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aruoma, O.I.; Halliwell, B. Free Radicals and Food Additives; Taylor & Francis: London, UK, 1991. [Google Scholar]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Brach, M.A.; De Vos, S.; Arnold, C.; Gruß, H.J.; Mertelsmann, R.; Herrmann, F. Leukotriene B4 transcriptionally activates interleukin-6 expression involving NK-xB and NF-IL6. Eur. J. Immunol. 1992, 22, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Stankova, J.; Rola-Pleszczynski, M. Leukotriene B4 stimulates c-fos and c-jun gene transcription and AP-1 binding activity in human monocytes. Biochem. J. 1992, 282, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.D.; Lau, W.; Kwiat, G.; Goetzl, E.J. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science 1984, 225, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Rafa, H.; Benkhelifa, S.; AitYounes, S.; Saoula, H.; Belhadef, S.; Belkhelfa, M.; Boukercha, A.; Toumi, R.; Soufli, I.; Moralès, O.; et al. All-Trans Retinoic Acid Modulates TLR4/NF-κB Signaling Pathway Targeting TNF-α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa during Ulcerative Colitis and Colitis Associated Cancer. Mediat. inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Toumi, R.; Soufli, I.; Rafa, H.; Belkhelfa, M.; Biad, A.; Touil-Boukoffa, C. Probiotic Bacteria Lactobacillus and Bifidobacterium Attenuate Inflammation in Dextran Sulfate Sodium-Induced Experimental Colitis in Mice. Int. J. Immunopath. Ph. 2014, 27, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.A.; Borges, F.; Ferreira, M.A. Effects of phenolic propyl esters on the oxidative stability of refined sunflower oil. J. Agric. Food Chem. 2001, 49, 3936–3941. [Google Scholar] [CrossRef]
- Chung, W.Y.; Jung, Y.J.; Surh, Y.J.; Lee, S.S.; Park, K.K. Antioxidative and antitumor promoting effects of [6]-paradol and its homologs. Mutat. Res. 2001, 496, 199–206. [Google Scholar] [CrossRef]
- Gomes, C.A.; da Cruz, T.G.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M.P. Anticancer activity of phenolic acids of natural or synthetic origin: A structure-activity study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girao da Cruz, M.T.; Cordeiro, M.N.; Milhazes, N.; Borges, F.; Marques, M.P. Phenolic acid derivatives with potential anticancer properties—A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niki, E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012, 586, 3767–3770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. Med. Chem. 2006, 2, 251–264. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Geromichalos, G.; Papageorgiou, A. Anticancer activity and quantitative-structure activity relationship (QSAR) studies of a series of antioxidant/anti-inflammatory aryl-acetic and hydroxamic acids. Chem. Biol. Drug Des. 2009, 74, 266–275. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martinez, P.; Medina-Remon, A.; Lamuela-Raventos, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, S.; Ajaz Rasool, S.; Asad Sayeed, S.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol.-Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef] [PubMed]
- Benfeito, S.; Oliveira, C.; Soares, P.; Fernandes, C.; Silva, T.; Teixeira, J.; Borges, F. Antioxidant therapy: Still in search of the ‘magic bullet’. Mitochondrion 2013, 13, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.; Siquet, C.; Orru, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef]
- Bayer, T.; Chakrabarti, A.; Lancelot, J.; Shaik, T.B.; Hausmann, K.; Melesina, J.; Schmidtkunz, K.; Marek, M.; Erdmann, F.; Schmidt, M.; et al. Synthesis, Crystallization Studies, and in vitro Characterization of Cinnamic Acid Derivatives as SmHDAC8 Inhibitors for the Treatment of Schistosomiasis. ChemMedChem 2018, 13, 1517–1529. [Google Scholar] [CrossRef]
- Rekker, R. Hydrophobic Fragmental Constant; Elsevier Scientific Co.: Amsterdam, The Netherlands, 1977; Volume 1, p. 19. [Google Scholar]
- Bate-Smith, E.C.; Westall, R.G. Chromatographic behavior and chemical structure in some naturally occurring phenolic substances. Biochim. Biophys. Acta 1950, 4, 427–440. [Google Scholar] [CrossRef]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.L. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radic. Res. 1996, 25, 369–384. [Google Scholar] [CrossRef]
- Betigeri, S.; Thakur, A.; Raghavan, K. Use of 2,2′-azobis(2-amidinopropane) dihydrochloride as a reagent tool for evaluation of oxidative stability of drugs. Pharm. Res. 2005, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic determination of the active site iron and its ligands in soybean lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Jankun, E.; Amzel, L.M.; Kroa, B.A.; Funk, M.O. Structure of soybean lipoxygenase L3 and a comparison with its L1 isoenzyme. Proteins 1997, 29, 15–31. [Google Scholar] [CrossRef]
- Chruszcz, M.; Wlodawer, A.; Minor, W. Determination of protein structures—A series of fortunate events. Biophys. J. 2008, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Lipoxygenase inhibitors: A comparative QSAR study review and evaluation of new QSARs. Med. Res. Rev. 2008, 28, 39–117. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T.; Afanas’ev, I.B.; Denisova, T.; Drozdova, T.; Trepalin, S. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor and Francis: Boca Raton, FL, USA, 2005; p. 1024. [Google Scholar]
- Biobyte Corp. C-QSAR Database. Available online: www.biobyte.com (accessed on 19 December 2018).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fiser, A.; Sali, A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Compd. | Clog P a | RM b (±SD) c | LOX d,e IC50 (μM) (% Inhibition 100 µM) | RA% 50 µM 20 min | RA% 50 µM 60 min | RA% 100 µM 20 min | RA% 100 µM 60 min |
|---|---|---|---|---|---|---|---|
| 1i | 6.25 | −0.103 (0.009) | 100 | 38 | 37 | 44 | 46 |
| 1ii | 4.72 | −0.537 (0.002) | 91.5 | 40 | 39 | 45 | 47 |
| 2i | 7.00 | 0.605 (0.016) | 76.0 | 35 | 34 | 37 | 40 |
| 2ii | 5.47 | 0.061 (0.007) | 77.0 | 36 | 37 | 43 | 56 |
| 3i | 5.07 | 0.582 (0.037) | 7.4 | 34 | 37 | 48 | 51 |
| 3ii | 3.54 | −0.642 (0.032) | 89.0 | 43 | 35 | 44 | 46 |
| 4i | 5.40 | 0.423 (0.017) | 28% | 30 | 30 | 38 | 36 |
| 4ii | 3.87 | 0.011 (0.001) | 69.0 | 34 | 46 | 35 | 37 |
| 5i | 5.30 | 0.683 (0.015) | 77.5 | 33 | 33 | 36 | 38 |
| 5ii | 3.77 | −0.565 (0.003) | 81.5 | 32 | 39 | 30 | 41 |
| 6i | 7.33 | 0.480 (0.035) | 60.0 | 38 | 36 | 44 | 39 |
| 6ii | 5.80 | 0.622 (0.025) | 74.0 | 43 | 36 | 31 | 36 |
| 7i | 7.24 | 1.151 (0.021) | 72.5 | 34 | 35 | 30 | 31 |
| 7ii | 5.71 | 0.654 (0.016) | 57.5 | 33 | 35 | 33 | 34 |
| NDGA | - | - | 0.45 | 81 | 83 | 87 | 93 |
| Compd. | HO•% a 100 µM | O2−•% a 100 µM | ABTS+•% a 100 µM | AAPH% a 100 µM | AAPH% a 50 µM | Docking Scores |
|---|---|---|---|---|---|---|
| 1i | 94 | 54 | 29 | 90 | 34 | −9.5 |
| 1ii | 100 | 8 | 83 | 94 | 22 | −8.0 |
| 2i | 95 | na | 32 | 96 | 48 | −9.5 |
| 2ii | 99 | na | 47 | 87 | 49 | −9.2 |
| 3i | 100 | na | 83 | 88 | 43 | −7.2 |
| 3ii | 99 | 85 | 63 | 95 | 71 | −7.4 |
| 4i | 83 | 46 | 24 | 85 | 52 | −6.6 |
| 4ii | 96 | 100 | 24 | 97 | 59 | −8.6 |
| 5i | 82 | na | 30 | 98 | 10 | −7.3 |
| 5ii | 89 | 8 | 35 | 93 | 53 | −7.0 |
| 6i | 100 | na | 53 | 100 | 56 | −7.2 |
| 6ii | 100 | na | 38 | 96 | 64 | −6.9 |
| 7i | 87 | 54 | 28 | 100 | 61 | −7.2 |
| 7ii | 72 | 69 | 34 | 100 | 69 | −9.3 |
| Trolox | 73 | - | 88 | 93 | - | - |
| CA | 23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pontiki, E.; Hadjipavlou-Litina, D. Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies. Molecules 2019, 24, 12. https://doi.org/10.3390/molecules24010012
Pontiki E, Hadjipavlou-Litina D. Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies. Molecules. 2019; 24(1):12. https://doi.org/10.3390/molecules24010012
Chicago/Turabian StylePontiki, Eleni, and Dimitra Hadjipavlou-Litina. 2019. "Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies" Molecules 24, no. 1: 12. https://doi.org/10.3390/molecules24010012
APA StylePontiki, E., & Hadjipavlou-Litina, D. (2019). Multi-Target Cinnamic Acids for Oxidative Stress and Inflammation: Design, Synthesis, Biological Evaluation and Modeling Studies. Molecules, 24(1), 12. https://doi.org/10.3390/molecules24010012