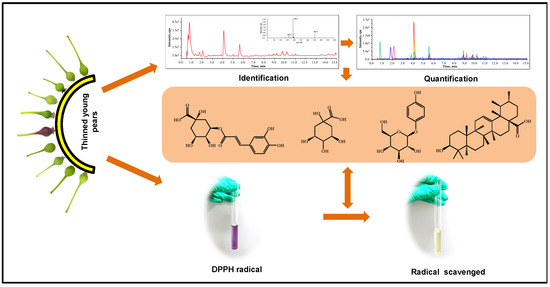
Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Polyphenol and Triterpenoid Composition
2.2. Quantification of Polyphenols and Triterpenoids
2.3. Antioxidant Capacity of Thinned Pears
2.4. Hierarchical Clustering Analysis
2.5. Correlation Analysis
3. Materials and Methods
3.1. Reagents and Standards
3.2. Materials
3.3. Extract Preparation
3.4. UPLC-Q TRAP-MS/MS Analysis of Polyphenols and Triterpenoids
3.4.1. UPLC Analysis
3.4.2. Identification of Bioactive Compounds in IDA Mode
3.4.3. Quantification of Bioactive Compounds in MRM Mode
3.4.4. Validation of Methodology
3.5. Analysis of Antioxidant Capacity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wouters, N.; De Ketelaere, B.; Deckers, T.; De Baerdemaeker, J.; Saeys, W. Multispectral detection of floral buds for automated thinning of pear. Comput. Electron. Agric. 2015, 113, 93–103. [Google Scholar] [CrossRef]
- Mazzola, M. Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. Phytopathology 1998, 88, 930–938. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, S.H.; Kim, E.H.; Yun, H.R.; Jeong, H.Y.; Lee, Y.G.; Kim, W.S.; Moon, J.H. Change in chemical constituents and free radical-scavenging activity during Pear (Pyrus pyrifolia) cultivar fruit development. Biosci. Biotechnol. Biochem. 2015, 79, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J. Funct. Foods 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.G.C.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B. Fast detection of phenolic compounds in extracts of easter pears (Pyrus communis) from the atacama desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC–Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef]
- Muffler, K.; Leipold, D.; Scheller, M.C.; Haas, C.; Steingroewer, J.; Bley, T.; Neuhaus, H.E.; Mirata, M.A.; Schrader, J.; Ulber, R. Biotransformation of triterpenes. Process Biochem. 2011, 46, 1–15. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Q.; Wang, M.; Fu, S.; Zhang, Q.; Zhang, Z.; Zhao, H.; Liu, Y.; Huang, Z.; Xie, Z.; et al. Using UHPLC Q-Trap/MS as a complementary technique to in-depth mine UPLC Q-TOF/MS data for identifying modified nucleosides in urine. J. Chromatogr. B 2017, 1051, 108–117. [Google Scholar] [CrossRef]
- Zuo, Y. High-Performance Liquid Chromatography (HPLC): Principles, Procedures and Practices; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- Kasote, D.M.; Ghosh, R.; Chung, J.Y.; Kim, J.; Bae, I.; Bae, H. Multiple reaction monitoring mode based liquid chromatography-mass spectrometry method for simultaneous quantification of brassinolide and other plant hormones involved in abiotic stresses. Int. J. Anal. Chem. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC-PDA-Q/TOF-MS. J. Food. Compost. Anal. 2016, 49, 65–77. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Fotirić Akšić, M.M.; Dabić, D.Č.; Gašić, U.M.; Zec, G.N.; Vulić, T.B.; Tešić, Ž.L.; Natić, M.M. Polyphenolic profile of pear leaves with different resistance to pear psylla (Cacopsylla pyri). J. Agric. Food Chem. 2015, 63, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Oszmianski, J.; Wojdylo, A. Increased content of phenolic compounds in pear leaves after infection by the pear rust pathogen. Physiol. Mol. Plant Pathol. 2015, 91, 113–119. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef]
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef]
- Gattuso, G.; Caristi, C.; Gargiulli, C.; Bellocco, E.; Toscano, G.; Leuzzi, U. Flavonoid glycosides in bergamot juice (Citrus bergamia Risso). J. Agric. Food Chem. 2006, 54, 3929–3935. [Google Scholar] [CrossRef]
- Benayad, Z.; Gomez-Cordoves, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Peng, L.Q.; Xu, J.J. Microcrystalline cellulose based matrix solid phase dispersion microextration for isomeric triterpenoid acids in loquat leaves by ultrahigh-performance liquid chromatography and quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2016, 1472, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, A.; Jóźwiak, G.; Waksmundzka-Hajnos, M. Simultaneous HPLC determination of pomolic, ursolic and euscaphic/tormentic acids in roots and rhizomes of various Potentilla species. Acta Chromatogr. 2014, 26, 97–110. [Google Scholar] [CrossRef]
- Verardo, G.; Gorassini, A.; Ricci, D.; Fraternale, D. High triterpenic acids production in callus cultures from fruit pulp of two apple varieties. Phytochem. Analysis 2017, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Long, P.; Sun, Y.; Meng, Q.; Liu, X.; Cui, H.; Lv, Q.; Zhang, L. The chemical profiling of loquat leaf extract by HPLC-DAD-ESI-MS and its effects on hyperlipidemia and hyperglycemia in rats induced by a high-fat and fructose diet. Food Funct. 2017, 8, 687–694. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crop. Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Galvis Sánchez, A.C.; Gil-Izquierdo, A.; Gil, M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003, 83, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Zuo, Y.; Lu, X.; Anwar, F.; Hameed, S. Characterization of free and conjugated phenolic compound in fruits of selected wild plants. Food Chem. 2016, 190, 80–89. [Google Scholar] [CrossRef]
- Willems, J.L.; Low, N.H. Structural identification of compounds for use in the detection of juice-to-juice debasing between apple and pear juices. Food Chem. 2018, 241, 346–352. [Google Scholar] [CrossRef]
- Du, G.R.; Li, M.J.; Ma, F.W.; Liang, D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Zhang, S. Pears; China Agriculture Press: Beijing, China, 2013. (In Chinese) [Google Scholar]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Qiao, Y.; Lin, S.; Jiang, Y.; Chen, F. Characterization of antioxidant compounds in Eriobotrya fragrans Champ leaf. Sci. Hortic. 2008, 118, 288–292. [Google Scholar] [CrossRef]
- Bai, X.; Lai, T.; Zhou, T.; Li, Y.; Li, X.; Zhang, H. In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect on A549 cell line. Molecules 2018, 23, 1395. [Google Scholar] [CrossRef] [PubMed]
- Lia, Z.J.; Wan, C.P.; Cai, L.; Li, S.Q.; Zheng, X.; Qi, Y.; Dong, J.W.; Yin, T.P.; Zhou, Z.X.; Tan, N.H.; et al. Terpenoids with cytotoxic activity from the branches and leaves of Pyrus pashia. Phytochem. Lett. 2015, 13, 246–251. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, Y. Ultrasound-assisted hydrolysis and gas chromatography-mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 2011, 128, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.-Y.; Gao, W.-Y.; Wang, Y.; Wang, H.-Y.; Cao, J.-G.; Huang, L.-Q. Chemical composition and anti-inflammatory and antioxidant activities of eight pear cultivars. J. Agric. Food Chem. 2012, 60, 8738–8744. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the thinned young pears reported in this study are available from the authors. |




| Peak NO. | Rt (min) | Formula | [M − H]− (m/z) | MS/MS Fragments (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| Polyphenol | |||||
| 1 | 0.81 | C7H12O6 | 191.1 | 172.9[M − H − H2O]− | Quinic acid a |
| 2 | 1.76 | C9H8O2 | 147.0 | 129.0[M − H − H2O]− | Cinnamic acid isomer |
| 3 | 2.06 | C12H16O7 | 317.1 # | 271.2[M − H]−, 160.9[M − H − C6H6O2]−, 109.0[M − H − Glc]− | Arbutin a |
| 4 | 2.77 | C30H24O12 | 575.3 | 449.0[M − H − C6H6O3]−, 285.2[M − H − C15H14O6]− | A-type procyanidin dimer |
| 5 | 3.19 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]−, 179.2[M − H − quinoyl]− | 4-O-Caffeoylquinic acid (Cryptochlorogenic acid) a |
| 6 | 3.37 | C15H20O9 | 343.2 | 181.0[M − H − hexose]− | Dihydro-caffeoyl-O-hexoside |
| 7 | 3.48 | C30H26O12 | 577.3 | 425.2[M − H − C8H8O3]−, 288.9[M − H − C15H12O6]− | B-type procyanidin dimer |
| 8 | 3.51 | C15H20O8 | 327.2 | 147.1[M − H − hexose − H2O]− | Hydroxyphenylpropionic acid-O-hexoside |
| 9 | 3.76 | C15H20O10 | 359.3 | 197.1[M − H − hexose]− | Syringic acid-O-hexoside |
| 10 | 3.84 | C12H14O6 | 253.1 | 135.1[M − H − C3H6O2 − CO2]− | Caffeoylglycerol |
| 11 | 3.88 | C15H20O9 | 343.2 | 181.0[M − H − hexose]− | Dihydro-caffeoyl-O-hexoside |
| 12 | 4.11 | C15H14O6 | 289.1 | 245.3[M − H − CO2]−, 202.9[M − H − H2O − C3O2]− | (+)-Catechin a |
| 13 | 4.13 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]−, 161.1[M − H − quinoyl − H2O]− | 3-O-Caffeoylquinic acid (Chlorogenic acid) a |
| 14 | 4.31 | C45H38O18 | 865.3 | 577.1[M − H − C15H12O6]−, 407.1[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 15 | 4.38 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]−, 173.0[M − H − caffeoyl − H2O]− | 5-O-Caffeoylquinic acid (Neochlorogenic acid) a |
| 16 | 4.64 | C22H18O10 | 441.3 | 325.1[M − H − C4H4O4]−, 163.0[M − H − C4H4O4 − caffeoyl]−, 118.9[p-coumaric acid − H − CO2]− | p-Coumaroylcaffeoyl malate |
| 17 | 4.89 | C30H26O12 | 577.3 | 425.1[M − H − C8H8O3]−, 407.0[M − H − C8H8O3 − H2O]−, 339.0[M − H − C8H8O3 − H2O − C3O2]−, 289.1[M − H − C15H12O6]−, 245.0[M − H − C15H12O6 − CO2]− | B-type procyanidin dimer |
| 18 | 4.98 | C45H38O18 | 865.3 | 577.0[M − H − C15H12O6]−, 287.1[M − H − C15H12O6 − C15H14O6]− | B-type procyanidin trimer |
| 19 | 5.07 | C45H36O18 | 863.3 | 573.3[M − H − C15H14O6]−, 289.2[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 20 | 5.28 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]− | 1-O-Caffeoylquinic acid |
| 21 | 5.30 | C15H18O9 | 341.1 | 178.9[M − H − hexose]− | Caffeoyl-O-hexoside |
| 22 | 5.44 | C16H16O8 | 335.3 | 179.1[M − H − shikimoyl]− | Caffeoylshikimic acid |
| 23 | 5.45 | C19H30O8 | 431.2 # | 385.0[M − H]−, 223.1[M − H − Glc]− | Roseoside |
| 24 | 5.48 | C15H14O6 | 289.1 | 245.0[M − H − CO2]−, 202.9[M − H − H2O − C3O2]− | (−)-Epicatechin a |
| 25 | 5.53 | C16H18O8 | 337.3 | 190.9[M − H − p-coumaroyl]−, 163.2 [M − H − quinoyl]− | 4-p-Coumaroylquinic acid |
| 26 | 5.57 | C15H20O10 | 359.3 | 197.0[M − H − hexose]−, 160.9[M − H − hexose − 2H2O]− | Syringic acid-O-hexoside |
| 27 | 5.64 | C45H36O18 | 863.3 | 711.3[M − H − C8H8O3]−, 573.0[M − H − C15H14O6]−, 289.0[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 28 | 6.05 | C16H16O8 | 335.3 | 179.0[M − H − shikimoyl]−, 135.1 [M − H − shikimoyl − CO2]− | Caffeoylshikimic acid |
| 29 | 6.49 | C45H36O18 | 863.3 | 711.1[M − H − C8H8O3]−, 573.0[M − H − C15H14O6]−, 451.2[M − H − C15H12O6 − C7H8O2]−, 289.2[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 30 | 6.54 | C30H24O12 | 575.3 | 285.3[M − H − C15H14O6]− | A-type procyanidin dimer |
| 31 | 6.69 | C17H20O9 | 367.4 | 191.0[M − H − feruloyl]−, 193.2[M − H − quinoyl]− | 3-O-Feruloylquinic acid |
| 32 | 6.71 | C19H16O12 | 435.3 | 273.0[M − H − caffeoyl]−, 205.0[M − H − caffeoylmalonyl]− | Caffeoyl-malonyl-methylcitric aci |
| 33 | 7.01 | C45H38O18 | 865.3 | 577.1[M − H − C15H12O6]−, 451.1[M − H − C15H12O6 − C6H6O3]−, 407.1[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 34 | 7.24 | C16H18O8 | 337.3 | 190.9[M − H − p-coumaroyl]−, 163.2 [M − H − quinoyl]− | 5-p-Coumaroylquinic acid |
| 35 | 7.35 | C30H26O12 | 577.3 | 289.3[M − H − C15H12O6]− | B-type procyanidin dimer |
| 36 | 7.40 | C17H20O9 | 367.4 | 205.1[M − H − caffeoyl]− | 4-O-Caffeoylquinic acid methyl ester |
| 37 | 7.58 | C30H24O12 | 575.3 | 285.2[M − H − C15H14O6]− | A-type procyanidin dimer |
| 38 | 7.61 | C32H38O20 | 741.4 | 301.1[M − H − Xyl-Rha-Gal]− | Quercetin-3-O-xylosylrhamnosylglucoside |
| 39 | 7.84 | C21H22O10 | 433.3 | 323.0[M − H − C6H6O2]−, 161.1[M − H − arbutin − H2O]− | Caffeoylarbutin |
| 40 | 7.89 | C60H50O24 | 1153.3 | 739.1[M − H − C15H12O6 − C6H6O2]−, 449.2[C30H26O12 − H − H2O − C6H6O2]−, 287.1[C30H26O12 − H − C15H14O6]− | B-type procyanidin tetramer |
| 41 | 8.04 | C24H24O13 | 519.4 | 315.3[M − H − acetyl-hexose]− | Isorhamnetin-acylated-hexoside |
| 42 | 8.05 | C21H22O10 | 433.3 | 323.0[M − H − C6H6O2]−, 178.9[M − H − arbutin]−, 160.9[M − H − arbutin − H2O]−, 133.1[M − H − arbutin − H2O − CO]− | Caffeoylarbutin |
| 43 | 8.11 | C26H28O16 | 595.4 | 301.1[M − H − Ara-Gal]− | Quercetin-3-O-arabinosylgalactoside |
| 44 | 8.13 | C45H36O18 | 863.3 | 573.1[M − H − C15H14O6]−, 451.1[M − H − C15H12O6 − C7H8O2]−, 289.2[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 45 | 8.19 | C30H24O12 | 575.3 | 448.9[M − H − C6H6O3]−, 285.1[M − H − C15H14O6]− | A-type procyanidin dimer |
| 46 | 8.32 | C15H20O10 | 359.3 | 197.2[M − H − hexose]− | Syringic acid-O-hexoside |
| 47 | 8.41 | C9H8O3 | 163.0 | 118.9[M − H − CO2]− | Hydroxycinnamic acid |
| 48 | 8.43 | C17H20O9 | 367.4 | 205.0[M − H − caffeoyl]− | 3-O-Caffeoylquinic acid methyl ester |
| 49 | 8.63 | C26H28O16 | 595.4 | 301.1[M − H − Ara-Glc]− | Quercetin-3-O-arabinosylglucoside |
| 50 | 8.65 | C27H30O16 | 609.4 | 301.0[M − H − Rha-Gal] | Quercetin-3-O-rhamnosylgalactoside |
| 51 | 8.72 | C21H20O11 | 447.3 | 285.2[M − H − Glc]− | Luteolin-7-O-galactoside |
| 52 | 8.87 | C27H30O16 | 609.4 | 301.0[M − H − Rut]− | Rutin a |
| 53 | 8.91 | C45H38O18 | 865.3 | 577.1[M − H − C15H12O6]−, 451.1[M − H − C15H12O6 − C6H6O3]−, 407.1[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 54 | 8.93 | C25H24O11 | 499.4 | 353.1[M − H − p-coumaroyl]−, 337.2[M − H − caffeoyl]−, 191.2[M − H − caffeoyl − p-coumaroyl]−, 163.1[p-coumaroyl − H]− | p-Coumaroylcaffeoylquinic acid |
| 55 | 8.98 | C21H20O12 | 463.2 | 301.2[M − H − Gal]− | Quercetin-3-O-galactoside (Hyperoside) a |
| 56 | 9.01 | C27H30O15 | 593.3 | 285.1[M − H − Rha-Gal]− | Kaempferol-3-O-rhamnosylgalactoside |
| 57 | 9.14 | C21H22O10 | 433.3 | 323.4[M − H − C6H6O2]−, 161.2[M − H − arbutin − H2O]−, 133.2[M − H − arbutin − H2O − CO]− | Caffeoylarbutin |
| 58 | 9.15 | C25H24O11 | 499.4 | 353. 1[M − H − p-coumaroyl]−, 336.9[M − H − caffeoyl]−, 191.1[M − H − caffeoyl − p-coumaroyl]−, 163.0[p-coumaroyl − H]− | p-Coumaroylcaffeoylquinic acid |
| 59 | 9.16 | C21H20O12 | 463.2 | 301.2[M − H − Glc]− | Quercetin-3-O-glucoside |
| 60 | 9.17 | C30H26O12 | 577.3 | 425.1[M − H − C8H8O3]−, 289.0[M − H − C15H12O6]− | B-type procyanidin dimer |
| 61 | 9.25 | C25H24O12 | 515.5 | 353.1[M − H − caffeoyl]−, 191.0[M − H − 2caffeoyl]− | Di-O-caffeoylquinic acid |
| 62 | 9.26 | C21H20O11 | 447.3 | 285.0[M − H − Glc]− | Luteolin-7-O-glucoside (Luteoloside) a |
| 63 | 9.30 | C45H38O18 | 865.3 | 577.2[M − H − C15H12O6]−, 406.9[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 64 | 9.38 | C27H30O15 | 593.3 | 285.1[M − H − Rha-Glc]− | Kaempferol-3-O-rhamnosylglucoside |
| 65 | 9.44 | C21H18O11 | 445.3 | 401.0[M − H − CO2]−, 357.2[M − H − 2CO2]−, 313.2[M − H − 3CO2]−, 225.2[M − H − C6H8O6 − CO2]−, 181.1[M − H − C6H8O6 − 2CO2]− | Apigenin-O-glucuronide or isomer |
| 66 | 9.46 | C30H24O12 | 575.3 | 539.0[M − H − H2O − H2O]−, 449.1[M − H − C6H6O3]−, 407.1[M − H − CO2 − C7H8O2]−, 285.1[M − H − C15H14O6]− | A-type procyanidin dimer |
| 67 | 9.51 | C45H34O18 | 861.3 | 735.1[M − H − C6H6O3]−, 693.2[M − H − CO2 − C7H8O2]−, 571.2[M − H − C15H14O6]− | A-type procyanidin trimer |
| 68 | 9.54 | C21H22O9 | 417.2 | 307.3[M − H − C6H6O2]−, 163.1[M − H − arbutin]−, 145.1[M − H − arbutin − H2O]− | p-Coumaroylarbutin |
| 69 | 9.65 | C17H20O9 | 367.4 | 205.0[M − H − caffeoyl]−, 191.1[M − H − caffeoyl − methyl]− | 5-O-Caffeoylquinic acid methyl ester |
| 70 | 9.69 | C45H36O18 | 863.3 | 575.0[M − H − C15H12O6]−, 449.0[M − H − C15H12O6 − C6H6O3]− | A-type procyanidin trimer |
| 71 | 9.70 | C19H16O12 | 435.3 | 273.1[M − H − caffeoyl]−, 205.3[M − H − caffeoylmalonyl]−, 161.1[M − H − caffeoylmalonyl − CO2]− | Caffeoyl-malonyl-methylcitric acid |
| 72 | 9.75 | C21H18O11 | 445.3 | 401.2[M − H − CO2]−, 357.2[M − H − 2CO2]−, 313.1[M − H − 3CO2]−, 225.1[M − H − C6H8O6 − CO2]− | Apigenin-O-glucuronide or isomer |
| 73 | 9.76 | C27H30O15 | 593.3 | 285.1[M − H − Rut]− | Kaempferol-3-O-rutinoside a |
| 74 | 9.78 | C23H22O13 | 505.3 | 445.2[M − H − CH3COOH]−, 301.1[M − H − acetyl-Gal]− | Quercetin-acylated-galactoside |
| 75 | 9.80 | C28H32O16 | 623.5 | 315.0[M − H − Gal-Rha]− | Isorhamnetin-3-O-rhamnosylgalactoside |
| 76 | 9.87 | C25H24O12 | 515.5 | 353.0[M − H − caffeoyl]− | 3,4-O-Dicaffeoylquinic acid (Isochlorogenic acid B) a |
| 77 | 9.92 | C27H30O14 | 577.3 | 269.1[M − H − Rut]− | Apigenin rutinoside |
| 78 | 9.95 | C28H32O16 | 623.5 | 315.0[M − H − Rut]− | Isorhamnetin-3-O-rutinoside a |
| 79 | 10.07 | C21H20O11 | 447.3 | 285.0[M − H − Glc]− | Kaempferol-3-O-galactoside |
| 80 | 10.09 | C23H22O13 | 505.3 | 445.1[M − H − CH3COOH]−, 301.1[M − H − acetyl-Glc]− | Quercetin-acylated-glucoside |
| 81 | 10.10 | C22H22O12 | 477.3 | 315.0[M − H − Gal]− | Isorhamnetin-3-O-galactoside |
| 82 | 10.14 | C28H32O15 | 607.4 | 299.1[M − H − Nhe]− | Chrysoeriol-7-neohesperidoside |
| 83 | 10.21 | C25H24O12 | 515.5 | 353.2[M − H − caffeoyl]−, 191.0[M − H − 2caffeoyl]−, 178.9[M − H − caffeoyl-quinoyl]− | 3,5-O-Dicaffeoylquinic acid (Isochlorogenic acid A) a |
| 84 | 10.22 | C30H24O12 | 575.3 | 406.9[M − H − CO2 − C7H8O2]− | A-type procyanidin dimer |
| 85 | 10.27 | C21H22O9 | 417.2 | 307.3[M − H − C6H6O2]−, 163.0[M − H − arbutin]−, 145.1[M − H − arbutin − H2O]− | p-Coumaroylarbutin |
| 86 | 10.28 | C22H22O12 | 477.3 | 315.0[M − H − Glc]− | Isorhamnetin-3-O-glucoside |
| 87 | 10.31 | C21H20O11 | 447.3 | 285.0[M − H − hexose]− | Kaempferol-3-O-glucoside |
| 88 | 10.34 | C21H20O10 | 431.2 | 269.1[M − H − hexose]− | Apigenin-O-hexoside |
| 89 | 10.38 | C25H24O11 | 499.4 | 353. 0[M − H − p-coumaroyl]−, 337.0[M − H − caffeoyl]−, 191.0[M − H − caffeoyl − p-coumaroyl]− | p-Coumaroylcaffeoylquinic acid |
| 90 | 10.58 | C22H22O11 | 461.4 | 298.9[M − H − Gal]− | Chrysoeriol-7-O-galactoside |
| 91 | 10.60 | C45H34O18 | 861.3 | 735.0[M − H − C6H6O3]−, 693.1[M − H − CO2 − C7H8O2]−, 571.1[M − H − C15H14O6]−, 288.9[M − H − 2C15H10O6]− | A-type procyanidin trimer |
| 92 | 10.71 | C25H24O12 | 515.5 | 353.1[M − H − caffeoyl]−, 191.1[M − H − 2caffeoyl]−, 179.0[M − H − caffeoyl-quinoyl]− | 4,5-O-Dicaffeoylquinic acid (Isochlorogenic acid C) a |
| 93 | 10.75 | C22H22O11 | 461.4 | 299.2[M − H − Glc]− | Chrysoeriol-7-O-glucoside |
| 94 | 10.80 | C23H22O12 | 489.1 | 285.1[M − H − acetyl-Gal]− | Kaempferol-acylated-galactoside |
| 95 | 10.85 | C24H24O13 | 519.4 | 315.3[M − H − acetyl-Gal]−, 299.2[M − H − acetyl-Gal − CH4]− | Isorhamnetin-acylated-galactoside |
| 96 | 11.05 | C24H24O13 | 519.5 | 315.0[M − H − acetyl-Glc]−, 299.2[M − H − acetyl-Glc − CH4]−, 271.2[M − H − acetyl-Glc − CH4 − H2O]− | Isorhamnetin-acylated-glucoside |
| 97 | 11.30 | C25H24O11 | 499.4 | 353. 0[M − H − p-coumaroyl]−, 337.1[M − H − caffeoyl]−, 190.9[M − H − caffeoyl − p-coumaroyl]−, 163.0[p−coumaroyl − H]− | p-Coumaroylcaffeoylquinic acid |
| 98 | 11.78 | C23H22O12 | 489.1 | 285.1[M − H − acetyl-Glc]− | Kaempferol-acylated-glucoside |
| 99 | 11.80 | C17H20O9 | 367.4 | 205.1[M − H − caffeoyl]−, 190.9[M − H − caffeoyl-methyl]−, 147.0[M − H − caffeoyl-methyl − CO2]− | 1-O-Caffeoylquinic acid methyl ester |
| 100 | 12.13 | C21H20O11 | 447.3 | 295.4[M − H − Galloyl]− | Galloyl-coumaric acid pentoside |
| 101 | 12.15 | C22H22O12 | 477.3 | 273.1[M − H − acetyl-hexose]− | Phloretin-acylated-hexoside |
| 102 | 12.38 | C21H20O11 | 447.3 | 295.1[M − H − Galloyl]− | Galloyl-coumaric acid pentoside |
| Triterpenoid | |||||
| 1′ | 1.21 | C30H48O5 | 487.3 | 469.1[M − H − H2O]−, 425.0[M − H − H2O − CO2]− | Euscaphic acid |
| 2′ | 1.40 | C30H48O5 | 487.3 | 469.3[M − H − H2O]−, 425.2[M − H − H2O − CO2]− | Tormentic acid |
| 3′ | 1.59 | C30H46O5 | 485.3 | 467.1[M − H − H2O]−, 423.2[M − H − H2O − CO2]− | Anmurcoic acid |
| 4′ | 1.95 | C30H48O4 | 471.3 | 453.2[M − H − H2O]−, 407.0[M − H − H2O − CH2O]− | Pomolic acid isomer |
| 5′ | 2.47 | C30H46O4 | 469.3 | 425.1[M − H − CO2]−, 407.0[M − H − H2O − CO2]− | 1-Hydroxy-3-oxours-12-en-28-oic acid or isomer |
| 6′ | 2.63 | C30H48O4 | 471.3 | 453.3[M − H − H2O]−, 407.1[M − H − H2O − CH2O]− | Pomolic acid a |
| 7′ | 2.89 | - | 701.5 | 641.2, 555.1, 540.1, 481.1 | Unknown |
| 8′ | 3.11 | C30H48O4 | 471.3 | 453.2[M − H − H2O]−, 407.1[M − H − H2O − CH2O]− | Alphitolic acid a |
| 9′ | 3.45 | C30H48O4 | 471.3 | 453.0[M − H − H2O]−, 407.1[M − H − H2O − CH2O]− | Maslinic acid a |
| 10′ | 3.79 | C30H48O4 | 471.3 | 453.2[M − H − H2O]−, 407.3[M − H − H2O − CH2O]− | Corosolic acid |
| 11′ | 4.38 | C30H46O4 | 469.3 | 425.2[M − H − CO2]−, 407.1[M − H − CO2 − H2O]− | 1-Hydroxy-3-oxours-12-en-28-oic acid or isomer |
| 12′ | 4.79 | C30H46O4 | 469.3 | 425.1[M − H − CO2]−, 407.3[M − H − CO2 − H2O]− | 1-Hydroxy-3-oxours-12-en-28-oic acid or isomer |
| 13′ | 6.89 | C30H48O3 | 455.3 | 407.4[M − H − H2O − CH2O]− | Betulinic acid a |
| 14′ | 7.44 | C30H48O3 | 455.3 | 407.4[M − H − H2O − CH2O]− | Oleanolic acid a |
| 15′ | 7.95 | C30H48O3 | 455.3 | 407.2[M − H − H2O − CH2O]− | Ursolic acid a |
| 16′ | 8.37 | - | 687.4 | 627.1, 541.2, 526.2, 467.0 | Unknown |
| Analyte | Ion Transition | Calibration Curve | R2 | Linear Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| Quinic acid | 191 > 93 | y = 10.2 x + 0.06 | 0.9996 | 7843.1–784,313.6 | 13.1 | 78.4 |
| Arbutin | 317 > 161 | y = 19.9 x + 0.6 | 0.9999 | 3919.2–391,921.6 | 16.3 | 98.0 |
| (+)-Catechin | 289 > 289 | y = 270.9 x − 0.7 | 0.9997 | 73.5–14,703.9 | 2.5 | 7.4 |
| (−)-Epicatechin | 289 > 289 | y = 246.8 x + 1.7 | 0.9998 | 4.9–49,166.6 | 1.6 | 4.9 |
| Chlorogenic acid | 353 > 179 | y = 1.3 x − 1.1 | 0.9996 | 3923.1–196,156.8 | 65.4 | 392.3 |
| Cryptochlorogenic acid | 353 > 179 | y = 101.1 x + 30.0 | 0.9998 | 32.6–1302.6 | 10.9 | 32.6 |
| Neochlorogenic acid | 353 > 173 | y = 128.7 x + 4.1 | 0.9999 | 25.7–1029.8 | 8.6 | 25.7 |
| Isochlorogenic acid A | 515 > 353 | y = 82.1 x − 0.9 | 0.9998 | 66.9–33,427.8 | 5.6 | 33.4 |
| Isochlorogenic acid B | 515 > 353 | y = 287.7 x + 69.3 | 0.9998 | 10.5–524.3 | 3.5 | 10.5 |
| Isochlorogenic acid C | 515 > 353 | y = 267.2 x + 43.2 | 0.9997 | 17.3–690.3 | 2.9 | 17.3 |
| p-Coumaric acid | 163 > 163 | y = 1270.4 x + 90.3 | 0.9998 | 2.0–9817.6 | 0.2 | 2.0 |
| Caffeic acid | 179 > 179 | y = 165.7 x + 15.4 | 0.9998 | 9.8–98,352.8 | 1.6 | 9.8 |
| Rutin | 609 > 300 | y = 365.1 x + 2.1 | 0.9999 | 19.7–4912.7 | 1.6 | 9.8 |
| 609 > 609 | y = 478.3 x + 53.1 | 0.9998 | 4.9–9825.5 | 1.6 | 4.9 | |
| Kaempferol-3-O-rutinoside | 593 > 285 | y = 36.2 x + 0.7 | 0.9997 | 49.4–4941.2 | 19.8 | 49.4 |
| 593 > 593 | y = 90.2 x + 2.3 | 0.9997 | 19.8–4941.2 | 9.9 | 19.8 | |
| Isorhamnetin-3-O-rutinoside | 623 > 315 | y = 136.3 x + 5.3 | 0.9998 | 20.0–9982.3 | 5.0 | 20.0 |
| 623 > 623 | y = 342.7 x − 12.9 | 0.9999 | 10.0–9982.3 | 5.0 | 10.0 | |
| Luteoloside | 447 > 285 | y = 646.2 x + 95.2 | 0.9997 | 5.0–981.2 | 1.6 | 4.9 |
| 447 > 447 | y = 504.6 x − 11.0 | 0.9998 | 5.0–981.2 | 1.6 | 4.9 | |
| Hyperoside | 463 > 300 | y = 518.6 x + 20.7 | 0.9995 | 9.8–978.6 | 3.3 | 9.8 |
| Oleanic acid | 455 > 455 | y = 2546.2 x − 63.3 | 0.9999 | 9.8–982.2 | 0.8 | 2.0 |
| Ursolic acid | 455 > 455 | y = 2329.5 x + 44.9 | 0.9999 | 0.8–987.6 | 0.3 | 0.8 |
| Betulinic acid | 455 > 455 | y = 4256.9 x − 540.1 | 1.0000 | 0.8–489.7 | 0.3 | 0.8 |
| Pomolic acid | 471 > 471 | y = 1302.8 x − 22.8 | 0.9999 | 2.0–198.3 | 0.8 | 2.0 |
| Maslinic acid | 471 > 471 | y = 1255.5 x − 2.9 | 0.9999 | 2.0–493.1 | 0.8 | 2.0 |
| Corosolic acid | 471 > 471 | y = 1196.7 x − 8.6 | 0.9999 | 2.0–493.6 | 0.8 | 2.0 |
| Peak NO. | Compound | The Compound Content in Different Pear Varieties (μg/g FW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSSL | YL | CG | FS | NGL | XSL | KELXL | XJSL | HQL | SJL | Mean | ||
| Phenolic acid | ||||||||||||
| 1 | Quinic acid | 8798.1 ± 122.4 | 9532.7 ± 74.3 | 8788.1 ± 74.6 | 8892.3 ± 85.8 | 10,500.5 ± 93.4 | 8984.8 ± 68.8 | 6725.8 ± 44.4 | 6064.2 ± 49.4 | 4991.7 ± 49.5 | 8666.34 ± 103.37 | 8194.5 |
| 2 | Cinnamic acid isomer | 120.0 ± 1.7 | 153.2 ± 2.6 | 130.7 ± 1.9 | 144.4 ± 2.1 | 60.4 ± 0.6 | 90.4 ± 0.8 | 56.3 ± 0.8 | 30.9 ± 0.6 | 217.4 ± 4.2 | 203.8 ± 1.9 | 120.8 |
| 5 | Cryptochlorogenic acid | 5.3 ± 0.1 | 10.5 ± 0.2 | 5.7 ± 0.1 | 4.0 ± 0.1 | 2.6 ± 0.1 | 4.5 ± 0.1 | 6.7 ± 0.1 | 6.8 ± 0.1 | 8.3 ± 0.2 | 6.3 ± 0.2 | 6.1 |
| 10 | Caffeoylglycerol | 4.6 ± 0.1 | N.Q. | 19.9 ± 0.4 | 14.4 ± 0.2 | N.Q. | 5.4 ± 0.1 | 9.12 ± 0.1 | 4.2 ± 0.1 | 25.6 ± 0.2 | 31.2 ± 0.3 | 11.5 |
| 13 | Chlorogenic acid | 3988.5 ± 33.9 | 3816.4 ± 50.6 | 1783.6 ± 16.2 | 1162.7 ± 9.1 | 4139.0 ± 39.9 | 3760.7 ± 28.2 | 1207.7 ± 14.3 | 2311.6 ± 17.1 | 2564.1 ± 28.1 | 2485.3 ± 23.5 | 2722.0 |
| 15 | Neochlorogenic acid | 2.8 ± 0.1 | 3.4 ± 0.1 | 1.6 ± 0.1 | 1.0 ± 0.0 | 2.1 ± 0.0 | 3.2 ± 0.1 | 1.7 ± 0.0 | 2.8 ± 0.0 | 1.8 ± 0.1 | 2.0 ± 0.0 | 2.2 |
| 16 | p-Coumaroylcaffeoyl malate | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.Q. | 5.6 ± 0.1 | 10.8 ± 0.1 | 8.7 ± 0.2 | 2.5 |
| 20 | 1-O-Caffeoylquinic acid | 26.2 ± 0.3 | 22.4 ± 0.3 | 12.2 ± 0.2 | 14.8 ± 0.2 | 20.5 ± 0.2 | 28.2 ± 0.3 | 10.9 ± 0.1 | 24.0 ± 0.3 | 23.2 ± 0.2 | 24.3 ± 0.2 | 20.7 |
| 22 | Caffeoylshikimic acid | 1.0 ± 0.0 | N.Q. | 0.9 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 2.0 ± 0.0 | 0.8 ± 0.0 | 1.8 ± 0.1 | 1.4 ± 0.0 | 1.0 |
| 25 | 4-p-Coumaroylquinic acid | 28.1 ± 0.2 | 32.4 ± 0.4 | 5.2 ± 0.1 | 3.0 ± 0.1 | 22.5 ± 0.5 | 17.2 ± 0.2 | 4.8 ± 0.1 | 8.6 ± 0.1 | 34.3 ± 0.5 | 22.4 ± 0.2 | 17.8 |
| 28 | Caffeoylshikimic acid | 25.7 ± 0.2 | 19.0 ± 0.2 | 32.8 ± 0.5 | 20.4 ± 0.2 | 13.5 ± 0.2 | 27.6 ± 0.3 | 7.4 ± 0.2 | 5.0 ± 0.1 | 8.3 ± 0.1 | 12.5 ± 0.1 | 17.2 |
| 31 | 3-O-Feruloylquinic acid | 49.9 ± 0.6 | 41.2 ± 0.5 | 12.8 ± 0.2 | 5.0 ± 0.1 | 49.6 ± 0.8 | 51.8 ± 0.9 | 6.5 ± 0.1 | 9.8 ± 0.2 | 19.9 ± 0.3 | 53.2 ± 0.9 | 30.0 |
| 32 | Caffeoyl-malonyl-methylcitric acid | 4.9 ± 0.1 | 5.3 ± 0.1 | 1.7 ± 0.0 | 0.5 ± 0.0 | 7.2 ± 0.1 | 6.4 ± 0.1 | 0.7 ± 0.0 | 1.2 ± 0.0 | 2.3 ± 0.0 | 6.3 ± 0.1 | 3.7 |
| 34 | 5-p-Coumaroylquinic acid | 1.0 ± 0.0 | 1.1 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 3.1 ± 0.0 | 2.3 ± 0.1 | 1.1 |
| 36 | 4-O-Caffeoylquinic acid methyl ester | 1.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.4 ± 0.0 | 2.4 ± 0.0 | 2.3 ± 0.0 | 1.4 ± 0.1 | 1.4 ± 0.0 | 6.4 ± 0.2 | 7.7 ± 0.2 | 2.7 |
| 47 | Hydroxycinnamic acid | 1.1 ± 0.0 | N.D. | 1.4 ± 0.0 | 1.7 ± 0.0 | 1.2 ± 0.0 | 1.5 ± 0.0 | 0.6 ± 0.0 | 5.7 ± 0.1 | 3.4 ± 0.0 | 1.9 ± 0.0 | 1.8 |
| 48 | 3-O-Caffeoylquinic acid methyl ester | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.Q. | 5.0 ± 0.1 | 5.1 ± 0.1 | 1.0 |
| 54 | p-Coumaroylcaffeoylquinic acid | N.D. | N.D. | N.D. | N.D. | 1.3 ± 0.0 | N.Q. | 0.6 ± 0.0 | N.D. | N.D. | N.D. | 0.2 |
| 58 | p-Coumaroylcaffeoylquinic acid | N.D. | N.D. | N.D. | N.D. | 4.5 ± 0.1 | N.Q. | 2.1 ± 0.0 | N.Q. | N.D. | N.D. | 0.7 |
| 61 | Di-O-caffeoylquinic acid | 3.1 ± 0.1 | N.D. | N.D. | N.D. | 1.7 ± 0.0 | N.Q. | 3.0 ± 0.0 | N.Q. | N.D. | N.D. | 0.8 |
| 69 | 5-O-Caffeoylquinic acid methyl ester | 6.2 ± 0.2 | N.Q. | 1.7 ± 0.0 | 9.6 ± 0.2 | 44.1 ± 0.7 | N.Q. | 12.6 ± 0.2 | N.Q. | 17.5 ± 0.2 | 13.3 ± 0.2 | 10.5 |
| 71 | Caffeoyl-malonyl-methylcitric acid | 11.6 ± 0.2 | N.Q. | 3.9 ± 0.0 | 18.6 ± 0.2 | 87.7 ± 0.9 | 1.3 ± 0.0 | 23.1 ± 0.2 | N.Q. | 34.7 ± 0.3 | 26.2 ± 0.3 | 20.7 |
| 76 | Isochlorogenic acid B | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.0 | N.D. | N.Q. | 0.5 ± 0.0 | N.Q. | 0.5 ± 0.0 | N.Q. | 0.4 |
| 83 | Isochlorogenic acid A | 139.2 ± 2.2 | 240.8 ± 2.6 | 52.9 ± 1.3 | 34.8 ± 0.3 | 21.1 ± 0.3 | 60.4 ± 0.7 | 74.3 ± 0.8 | 52.8 ± 0.5 | 116.0 ± 1.4 | 89.2 ± 2.0 | 88.2 |
| 89 | p-Coumaroylcaffeoylquinic acid | 0.8 ± 0.0 | 0.6 ± 0.0 | N.D. | N.D. | 1.5 ± 0.0 | N.Q. | N.Q. | N.Q. | 1.1 ± 0.1 | 0.6 ± 0.0 | 0.5 |
| 92 | Isochlorogenic acid C | 3.2 ± 0.1 | 4.2 ± 0.1 | 2.8 ± 0.0 | 1.8 ± 0.1 | 2.0 ± 0.0 | 1.6 ± 0.0 | 2.4 ± 0.1 | 1.6 ± 0.0 | 1.6 ± 0.0 | 1.3 ± 0.0 | 2.3 |
| 97 | p-Coumaroylcaffeoylquinic acid | 17.2 ± 0.2 | 21.3 ± 0.2 | 4.8 ± 0.1 | 3.1 ± 0.1 | 2.3 ± 0.0 | 3.9 ± 0.1 | 8.1 ± 0.1 | 3.8 ± 0.0 | 18.2 ± 0.2 | 11.2 ± 0.2 | 9.4 |
| 99 | 1-O-Caffeoylquinic acid methyl ester | 1.2 ± 0.0 | N.Q. | N.Q. | 1.6 ± 0.0 | 5.4 ± 0.1 | N.Q. | 3.4 ± 0.1 | N.Q. | 6.8 ± 0.2 | 5.4 ± 0.1 | 2.4 |
| Total phenolic acids | 13,242.2 ± 117.8 | 13,905.9 ± 96.8 | 10,864.4 ± 86.4 | 10,337.2 ± 95.9 | 14,994.6 ± 99.2 | 13,052.6 ± 72.1 | 8172.6 ± 52.8 | 8541.3 ± 61.4 | 8123.9 ± 50.4 | 11,688.1 ± 89.6 | 11,292.3 | |
| Phenolic glycoside | ||||||||||||
| 3 | Arbutin | 3925.2 ± 70.1 | 5605.5 ± 53.9 | 4843.8 ± 80.4 | 4945.5 ± 78.1 | 5899.1 ± 71.3 | 5905.9 ± 60.1 | 3007.4 ± 49.4 | 2285.6 ± 22.3 | 3511.6 ± 57.3 | 4510.3 ± 31.6 | 4444.0 |
| 6 | Dihydro-caffeoyl-O-hexoside | 9.1 ± 0.2 | 47.6 ± 0.5 | 25.9 ± 0.2 | 3.8 ± 0.1 | 48.4 ± 0.6 | 1.9 ± 0.1 | 1.4 ± 0.0 | 3.3 ± 0.1 | 19.2 ± 0.4 | 12.2 ± 0.1 | 17.3 |
| 8 | Hydroxyphenylpropionic acid-O-hexoside | 2.5 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 1.3 ± 0.1 | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.6 |
| 9 | Syringic acid-O-hexoside | 3.8 ± 0.1 | N.Q. | 17.7 ± 0.2 | 12.7 ± 0.3 | N.Q. | 3.5 ± 0.1 | 7.6 ± 0.2 | 3.4 ± 0.1 | 21.7 ± 0.6 | 27.7 ± 0.5 | 9.8 |
| 11 | Dihydro-caffeoyl-O-hexoside | 8.8 ± 0.1 | 7.9 ± 0.1 | 4.9 ± 0.1 | 4.2 ± 0.1 | 8.7 ± 0.1 | 3.6 ± 0.1 | 10.1 ± 0.1 | 4.6 ± 0.1 | 2.8 ± 0.1 | 5.2 ± 0.1 | 6.1 |
| 21 | Caffeoyl-O-hexoside | 8.9 ± 0.1 | 12.9 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | 5.1 ± 0.1 | 5.6 ± 0.1 | 2.2 ± 0.0 | N.Q. | 3.8 ± 0.1 | 5.0 ± 0.1 | 5.3 |
| 23 | Roseoside | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.0 | 1.3 ± 0.0 | 0.5 |
| 26 | Syringic acid-O-hexoside | 1.4 ± 0.0 | 1.6 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 1.1 ± 0.0 | 0.8 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 1.8 ± 0.1 | 1.0 ± 0.0 | 0.9 |
| 39 | Caffeoylarbutin | N.D. | N.D. | N.D. | N.D. | N.Q. | N.D. | N.D. | 7.0 ± 0.1 | N.D. | 44.0 ± 0.5 | 5.1 |
| 42 | Caffeoylarbutin | 193.7 ± 2.7 | N.Q. | 201.1 ± 1.5 | 239.1 ± 2.5 | N.Q. | 74.1 ± 0.7 | 225.7 ± 1.7 | 126.9 ± 2.1 | N.Q. | 2.6 ± 0.1 | 106.3 |
| 46 | Syringic acid-O-hexoside | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.2 ± 0.0 | N.Q. | N.Q. | 0.2 ± 0.0 | 0.4 |
| 57 | Caffeoylarbutin | 9.9 ± 0.1 | N.Q. | 13.9 ± 0.2 | 14.9 ± 0.4 | N.Q. | 5.2 ± 0.1 | 13.5 ± 0.2 | 8.9 ± 0.2 | N.Q. | 2.8 ± 0.1 | 6.9 |
| 68 | p-Coumaroylarbutin | 33.9 ± 0.4 | 0.4 ± 0.0 | 34.8 ± 0.5 | 50.9 ± 0.8 | 1.0 ± 0.0 | 12.1 ± 0.2 | 39.7 ± 0.7 | 20.0 ± 0.2 | 0.4 ± 0.0 | 0.3 ± 0.0 | 19.3 |
| 85 | p-Coumaroylarbutin | 11.1 ± 0.2 | N.Q. | 14.0 ± 0.2 | 18.7 ± 0.4 | 0.3 ± 0.0 | 7.0 ± 0.1 | 17.4 ± 0.2 | 9.8 ± 0.1 | 1.3 ± 0.0 | 2.1 ± 0.1 | 8.2 |
| 100 | Galloyl-coumaric acid pentoside | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.6± 0.0 | 0.3 |
| 102 | Galloyl-coumaric acid pentoside | 0.1 ± 0.0 | 0.1 ± 0.00 | 0.1 ± 0.0 | N.Q. | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 |
| Total phenolic glycosides | 4209.2 ± 72.9 | 5678.1 ± 53.6 | 5162.8 ± 83.5 | 5296.0 ± 77.7 | 5966.6 ± 71.3 | 6021.3 ± 59.5 | 3326.3 ± 50.8 | 2471.0 ± 23.4 | 3564.4 ± 56.8 | 4616.0 ± 32.1 | 4631.2 | |
| Flavone | ||||||||||||
| 38 | Quercetin-3-O-xylosylrhamnosylglucoside | 1.7 ± 0.0 | 1.4 ± 0.0 | 2.6 ± 0.1 | 1.5 ± 0.0 | 1.1 ± 0.0 | 2.7 ± 0.0 | 4.8 ± 0.1 | N.Q. | N.D. | N.D. | 1.6 |
| 41 | Isorhamnetin-acylated-hexoside | 1.1 ± 0.0 | N.Q. | 1.1 ± 0.1 | 1.3 ± 0.0 | N.Q. | 0.4 ± 0.0 | 1.2 ± 0.0 | 0.7 ± 0.0 | N.D. | N.D. | 0.6 |
| 43 | Quercetin-3-O-arabinosylgalactoside | 5.9 ± 0.1 | 2.0 ± 0.1 | 5.1 ± 0.1 | 2.6 ± 0.1 | 1.0 ± 0.0 | 2.7 ± 0.1 | 3.4 ± 0.1 | N.Q. | N.D. | N.D. | 2.3 |
| 49 | Quercetin-3-O-arabinosylglucoside | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.1 |
| 50 | Quercetin-3-O-rhamnosylgalactoside | 0.3 ± 0.0 | 0.5 ± 0.0 | 1.0 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 2.2 ± 0.1 | 0.5 ± 0.0 | N.Q. | 16.9 ± 0.5 | 6.9 ± 0.1 | 2.9 |
| 51 | Luteolin-7-O-galactoside | N.D. | N.D. | N.D. | N.D. | 0.8 ± 0.0 | N.Q. | N.D. | N.D. | N.D. | N.D. | 0.1 |
| 52 | Rutin | 12.6 ± 0.1 | 10.1 ± 0.2 | 13.3 ± 0.2 | 8.3 ± 0.2 | 12.3 ± 0.2 | 19.7 ± 0.2 | 24.5 ± 0.4 | 1.6 ± 0.0 | 61.9 ± 1.1 | 61.2 ± 0.3 | 22.5 |
| 55 | Hyperoside | 2.7 ± 0.0 | 1.4 ± 0.0 | 2.0 ± 0.1 | 1.0 ± 0.0 | 0.4 ± 0.0 | 1.5 ± 0.0 | 0.4 ± 0.0 | 3.0 ± 0.1 | 11.4 ± 0.2 | 8.8 ± 0.1 | 3.2 |
| 56 | Kaempferol-3-O-rhamnosylgalactoside | 63.9 ± 1.7 | N.Q. | N.D. | N.D. | 12.1 ± 0.1 | N.Q. | 17.7 ± 0.3 | N.Q. | N.Q. | N.Q. | 9.4 |
| 59 | Quercetin-3-O-glucoside | 20.6 ± 0.2 | 7.9 ± 0.2 | 16.5 ± 0.3 | 7.7 ± 0.2 | 5.3 ± 0.1 | 12.3 ± 0.2 | 6.5 ± 0.1 | 16.1 ± 0.3 | 56.1 ± 0.7 | 50.4 ± 0.5 | 19.9 |
| 62 | Luteoloside | 14.1 ± 0.1 | N.Q. | N.D. | N.D. | 5.8 ± 0.1 | N.Q. | 11.5 ± 0.1 | N.Q. | N.D. | N.D. | 3.2 |
| 64 | Kaempferol-3-O-rhamnosylglucoside | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.95 ± 0.1 | 1.3 ± 0.1 | 0.3 |
| 65 | Apigenin-O-glucuronide or isomer | N.D. | N.D. | N.D. | N.D. | 0.2 ± 0.0 | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.1 |
| 72 | Apigenin-O-glucuronide or isomer | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | N.D. | 0.5 ± 0.0 | N.D. | N.D. | 3.3 ± 0.1 | 1.5 ± 0.0 | 0.7 |
| 73 | Kaempferol-3-O-rutinoside | 2.3 ± 0.1 | 1.7 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 1.2 ± 0.0 | 4.5 ± 0.2 | 4.0 ± 0.1 | 17.4 ± 0.5 | 15.9 ± 0.4 | 5.5 |
| 74 | Quercetin-acylated-galactoside | 5.98 ± 0.1 | 2.1 ± 0.0 | 3.3 ± 0.0 | 1.6 ± 0.0 | 0.9 ± 0.0 | 4.8 ± 0.1 | N.D. | N.D. | 39.4 ± 0.9 | 16.1 ± 0.2 | 7.4 |
| 75 | Isorhamnetin-3-O-rhamnosylgalactoside | 10.8 ± 0.2 | 21.3 ± 0.4 | 10.2 ± 0.2 | 7.3 ± 0.1 | 8.7 ± 0.1 | 2.3 ± 0.1 | 12.6 ± 0.4 | 15.8 ± 0.2 | 20.2 ± 0.2 | 58.8 ± 0.7 | 16.8 |
| 77 | Apigenin rutinoside | 3.8 ± 0.1 | N.Q. | N.D. | N.D. | 1.2 ± 0.0 | N.Q. | N.D. | N.D. | N.Q. | 1.0 ± 0.0 | 0.6 |
| 78 | Isorhamnetin-3-O-rutinoside | 86.9 ± 1.2 | 98.1 ± 1.1 | 49.9 ± 0.7 | 66.4 ± 1.0 | 75.3 ± 1.7 | 9.1 ± 0.1 | 136.6 ± 1.6 | 62.9 ± 1.4 | 28.5 ± 0.5 | 143.8 ± 1.3 | 75.8 |
| 79 | Kaempferol-3-O-galactoside | 4.3 ± 0.1 | 1.7 ± 0.0 | 8.2 ± 0.1 | 8.2 ± 0.2 | 1.6 ± 0.1 | 3.5 ± 0.1 | 2.1 ± 0.1 | 12.8 ± 0.3 | 16.8 ± 0.5 | 8.1 ± 0.2 | 6.7 |
| 80 | Quercetin-acylated-glucoside | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.6 ± 0.0 | N.Q. | N.Q. | 3.1 ± 0.0 | 2.1 ± 0.1 | 0.8 |
| 81 | Isorhamnetin-3-O-galactoside | 5.7 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 1.7 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.1 | 0.4 ± 0.0 | 19.8 ± 0.2 | 1.4 ± 0.0 | 4.6 ± 0.1 | 4.0 |
| 82 | Chrysoeriol-7-neohesperidoside | 9.3 ± 0.2 | N.Q. | N.Q. | N.Q. | 2.7 ± 0.1 | N.Q. | 2.0 ± 0.0 | N.Q. | N.Q. | N.Q. | 1.4 |
| 86 | Isorhamnetin-3-O-glucoside | 21.3 ± 0.4 | 8.8 ± 0.2 | 13.0 ± 0.3 | 9.2 ± 0.2 | 4.6 ± 0.1 | 2.4 ± 0.0 | 3.7 ± 0.1 | 95.1 ± 0.9 | 7.4 ± 0.2 | 22.1 ± 0.4 | 18.8 |
| 87 | Kaempferol-3-O-glucoside | 2.4 ± 0.1 | N.Q. | N.D. | 1.1 ± 0.1 | 55.1 ± 0.9 | N.Q. | 2.6 ± 0.1 | N.Q. | N.Q. | N.Q. | 6.1 |
| 88 | Apigenin-O-hexoside | 9.8 ± 0.2 | N.Q. | N.D. | N.D. | 19.6 ± 0.4 | N.Q. | 3.5 ± 0.1 | N.Q. | 0.6 ± 0.1 | 0.7 ± 0.0 | 3.4 |
| 90 | Chrysoeriol-7-O-galactoside | 15.7 ± 0.2 | N.Q. | N.D. | N.D. | 14.7 ± 0.2 | N.Q. | 19.9 ± 0.1 | N.Q. | N.Q. | N.Q. | 5.0 |
| 93 | Chrysoeriol-7-O-glucoside | 0.5 ± 0.0 | N.D. | N.D. | N.D. | 22.9 ± 0.6 | N.Q. | N.D. | N.D. | 0.2 ± 0.0 | 0.3 ± 0.0 | 2.4 |
| 94 | Kaempferol-acylated-galactoside | 14.5 ± 0.4 | 7.1 ± 0.2 | 6.3 ± 0.1 | 8.3 ± 0.2 | 4.0 ± 0.1 | 2.4 ± 0.1 | N.Q. | 2.0 ± 0.1 | 150.5 ± 2.1 | 28.0 ± 0.3 | 22.3 |
| 95 | Isorhamnetin-acylated-galactoside | 6.2 ± 0.1 | 2.95 ± 0.1 | 1.3 ± 0.0 | 1.1 ± 0.0 | N.D. | N.D. | N.D. | N.D. | 3.8 ± 0.1 | 3.3 ± 0.1 | 1.9 |
| 96 | Isorhamnetin-acylated-glucoside | 53.7 ± 1.1 | 26.1 ± 0.2 | 15.1 ± 0.2 | 14.6 ± 0.3 | 4.8 ± 0.1 | 2.0 ± 0.1 | N.Q. | 3.1 ± 0.0 | 36.5 ± 0.7 | 55.6 ± 0.9 | 21.2 |
| 98 | Kaempferol-acylated-glucoside | 2.8 ± 0.1 | N.Q. | N.D. | N.D. | N.D. | N.D. | 5.4 ± 0.1 | 1.3 ± 0.0 | N.Q. | 1.2 ± 0.1 | 1.1 |
| 101 | Phloretin-acylated-hexoside | 1.1 ± 0.0 | N.Q. | N.D. | 2.1 ± 0.1 | N.D. | N.D. | 3.3 ± 0.1 | N.D. | N.D. | N.D. | 0.6 |
| Total flavones | 380.9 ± 4.7 | 196.1 ± 0.3 | 154.7 ± 1.1 | 147.3 ± 1.5 | 259.0 ± 3.1 | 71.1 ± 1.4 | 266.9 ± 2.0 | 238.3 ± 3.6 | 478.1 ± 3.7 | 492.2 ± 1.6 | 268.5 | |
| Flavan-3-ol | ||||||||||||
| 4 | A-type procyanidin dimer | N.D. | N.D. | N.Q. | 0.7 ± 0.0 | N.D. | N.D. | N.Q. | 0.7 ± 0.0 | 3.3 ± 0.1 | 1.3 ± 0.0 | 0.6 |
| 7 | B-type procyanidin dimer | N.D. | N.D. | 0.9 ± 0.0 | 1.7 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 2.7 ± 0.1 | 6.8 ± 0.2 | 4.3 ± 0.1 | 1.8 |
| 12 | (+) -Catechin | 17.6 ± 0.3 | 16.5 ± 0.2 | 26.1 ± 0.4 | 40.1 ± 0.7 | 25.5 ± 0.4 | 12.9 ± 0.2 | 7.0 ± 0.1 | 26.6 ± 0.5 | 99.3 ± 1.3 | 134.3 ± 1.2 | 40.6 |
| 14 | B-type procyanidin trimer | N.D. | N.D. | 0.5 ± 0.0 | 1.6 ± 0.0 | N.D. | N.D. | N.Q. | 0.8 ± 0.0 | 3.2 ± 0.1 | 1.4 ± 0.0 | 0.7 |
| 17 | B-type procyanidin dimer | N.D. | N.D. | 2.2 ± 0.0 | 6.2 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.0 | 1.9 ± 0.0 | 5.98 ± 0.1 | 37.8 ± 0.7 | 8.3 ± 0.2 | 6.5 |
| 18 | B-type procyanidin trimer | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.9 ± 0.0 | N.Q. | 0.2 |
| 19 | A-type procyanidin trimer | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.Q. | 0.6 ± 0.0 | 1.6 ± 0.1 | 0.8 ± 0.0 | 0.3 |
| 24 | (-)-Epicatechin | 29.2 ± 0.5 | 18.3 ± 0.2 | 48.7 ± 0.9 | 85.7 ± 0.9 | 120.4 ± 1.7 | 40.0 ± 0.8 | 29.7 ± 0.4 | 69.8 ± 1.3 | 445.7 ± 7.2 | 219.6 ± 2.6 | 110.7 |
| 27 | A-type procyanidin trimer | 0.5 ± 0.0 | N.D. | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | N.Q. | N.Q. | 0.9 ± 0.1 | 1.7 ± 0.0 | 2.3 ± 0.1 | 0.7 |
| 29 | A-type procyanidin trimer | 0.7 ± 0.0 | N.D. | 1.7 ± 0.0 | 2.0 ± 0.0 | 2.9 ± 0.1 | 1.5 ± 0.1 | 1.1 ± 0.0 | 3.4 ± 0.1 | 10.0 ± 0.1 | 5.5 ± 0.1 | 2.9 |
| 30 | A-type procyanidin dimer | N.D. | N.D. | N.Q. | 0.6 ± 0.0 | N.D. | N.D. | N.Q. | 0.9 ± 0.0 | 3.2 ± 0.1 | 1.2 ± 0.0 | 0.6 |
| 33 | B-type procyanidin trimer | N.D. | N.D. | 0.6 ± 0.0 | 3.0 ± 0.0 | N.D. | N.D. | 0.6 ± 0.0 | 2.0 ± 0.0 | 15.2 ± 0.1 | 2.3 ± 0.0 | 2.4 |
| 35 | B-type procyanidin dimer | N.D. | N.D. | N.Q. | 0.9 ± 0.0 | N.D. | N.D. | N.Q. | 0.6 ± 0.0 | 2.1 ± 0.1 | 1.9 ± 0.1 | 0.6 |
| 37 | A-type procyanidin dimer | 0.5 ± 0.0 | N.D. | N.Q. | 1.0 ± 0.0 | 1.5 ± 0.0 | N.Q. | 1.2 ± 0.0 | 0.7 ± 0.0 | 4.0 ± 0.1 | 2.0 ± 0.1 | 1.1 |
| 40 | B-type procyanidin tetramer | N.D. | N.D. | N.D. | 0.7 ± 0.0 | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 2.7 ± 0.0 | 0.7 ± 0.0 | 0.5 |
| 44 | A-type procyanidin trimer | N.D. | N.D. | 0.7 ± 0.0 | 1.0 ± 0.0 | N.D. | N.D. | N.Q. | 1.1 ± 0.0 | 2.9 ± 0.1 | 2.1 ± 0.1 | 0.8 |
| 45 | A-type procyanidin dimer | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.1 ± 0.0 | 1.7 ± 0.0 | 3.0 ± 0.1 | 1.7 ± 0.0 | 1.1 ± 0.0 | 4.5 ± 0.1 | 3.6 ± 0.1 | 7.4 ± 0.1 | 3.0 |
| 53 | B-type procyanidin trimer | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.1 ± 0.0 | N.Q. | 0.1 |
| 60 | B-type procyanidin dimer | N.D. | N.D. | 0.9 ± 0.1 | 2.3 ± 0.0 | 1.0 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.0 | 1.7 ± 0.0 | 10.2 ± 0.3 | 3.2 ± 0.0 | 2.1 |
| 63 | B-type procyanidin trimer | N.D. | N.D. | N.D. | 0.6 ± 0.0 | N.D. | N.D. | N.D. | N.D. | 2.1 ± 0.1 | 0.5 ± 0.0 | 0.3 |
| 66 | A-type procyanidin dimer | 3.6 ± 0.1 | 2.1 ± 0.0 | 4.5 ± 0.0 | 5.2 ± 0.1 | 10.6 ± 0.2 | 4.8 ± 0.1 | 3.6 ± 0.1 | 8.6 ± 0.2 | 20.1 ± 0.2 | 14.4 ± 0.3 | 7.8 |
| 67 | A-type procyanidin trimer | 2.0 ± 0.0 | 1.7 ± 0.0 | 1.6 ± 0.1 | 0.8 ± 0.0 | 1.9 ± 0.0 | 1.0 ± 0.0 | N.Q. | 1.5 ± 0.0 | 1.4 ± 0.0 | 5.2 ± 0.1 | 1.7 |
| 70 | A-type procyanidin trimer | N.D. | N.D. | 1.0 ± 0.0 | 1.8 ± 0.0 | 1.3 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 2.6 ± 0.0 | 8.9 ± 0.1 | 4.3 ± 0.0 | 2.2 |
| 84 | A-type procyanidin dimer | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.1 ± 0.1 | 3.7 ± 0.0 | 5.7 ± 0.1 | 3.2 ± 0.0 | 2.3 ± 0.0 | 5.6 ± 0.1 | 16.1 ± 0.2 | 10.8 ± 0.1 | 5.6 |
| 91 | A-type procyanidin trimer | 3.6 ± 0.1 | 2.2 ± 0.1 | 2.9 ± 0.1 | 1.7 ± 0.0 | 10.6 ± 0.3 | 3.9 ± 0.1 | 1.9 ± 0.1 | 4.4 ± 0.1 | 6.7 ± 0.2 | 7.8 ± 0.2 | 4.6 |
| Total flavan-3-ols | 63.5 ± 0.1 | 46.1 ± 0.5 | 98.0 ± 0.4 | 163.8 ± 0.9 | 186.3 ± 2.3 | 72.1 ± 0.8 | 52.3 ± 0.6 | 146.1 ± 1.6 | 711.7 ± 8.5 | 441.2 ± 3.8 | 198.1 | |
| Total polyphenols (mg/g FW) | 17.9 ± 0.1 | 19.8 ± 0.1 | 16.3 ± 0.1 | 15.9 ± 0.2 | 21.4 ± 0.1 | 19.2 ± 0.1 | 11.8 ± 0.1 | 11.4 ± 0.1 | 12.9 ± 0.1 | 17.2 ± 0.1 | 16.4 | |
| Triterpenoid | ||||||||||||
| 1′ | Euscaphic acid | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 0.3 ± 0.0 | N.D. | 0.5 ± 0.0 | N.D. | N.D. | N.D. | 0.1 |
| 2′ | Tormentic acid | 6.0 ± 0.1 | 20.5 ± 0.5 | 14.5 ± 0.4 | 13.4 ± 0.3 | 11.6 ± 0.3 | 8.2 ± 0.2 | 5.3 ± 0.1 | 7.6 ± 0.1 | 15.8 ± 0.2 | 13.1 ± 0.2 | 11.6 |
| 3′ | Anmurcoic acid | 13.9 ± 0.2 | 55.3 ± 0.7 | 9.6 ± 0.1 | 18.0 ± 0.4 | 11.1 ± 0.1 | 8.7 ± 0.2 | 11.0 ± 0.2 | 6.7 ± 0.1 | 3.8 ± 0.1 | 9.3 ± 0.1 | 14.8 |
| 4′ | Pomolic acid isomer | 82.2 ± 1.2 | 60.7 ± 1.0 | 15.0 ± 0.3 | 25.5 ± 0.3 | 42.1 ± 0.8 | 2.9 ± 0.1 | 64.5 ± 1.2 | 50.0 ± 0.9 | 9.5 ± 0.2 | 81.1 ± 1.1 | 43.4 |
| 5′ | 1-Hydroxy-3-oxours-12-en-28-oic acid/isomer | 4.6 ± 0.1 | 7.3 ± 0.1 | N.D. | 0.5 ± 0.0 | 0.4 ± 0.0 | N.D. | 1.5 ± 0.0 | 2.3 ± 0.0 | N.D. | 1.2 ± 0.0 | 1.8 |
| 6′ | Pomolic acid | 6.6 ± 0.1 | 2.4 ± 0.0 | 1.8 ± 0.1 | 2.6 ± 0.1 | 1.9 ± 0.1 | 5.9 ± 0.1 | 4.5 ± 0.1 | 1.9 ± 0.0 | 2.7 ± 0.1 | 9.7 ± 0.2 | 4.0 |
| 7′ | 701 m/z [M − H]- | 6.1 ± 0.1 | 8.3 ± 0.2 | 5.2 ± 0.1 | 6.1 ± 0.2 | 5.3 ± 0.1 | 4.6 ± 0.1 | 4.9 ± 0.0 | 7.5 ± 0.2 | 13.4 ± 0.1 | 19.1 ± 0.3 | 8.1 |
| 8′ | Alphitolic acid | 1.4 ± 0.0 | 1.6 ± 0.0 | 0.4 ± 0.0 | 1.1 ± 0.0 | 0.9 ± 0.0 | 2.3 ± 0.0 | 2.7 ± 0.1 | 0.4 ± 0.0 | 0.9 ± 0.0 | 0.5 ± 0.0 | 1.2 |
| 9′ | Maslinic acid | 4.0 ± 0.1 | 28.5 ± 0.4 | 4.5 ± 0.1 | 10.9 ± 0.3 | 10.7 ± 0.2 | 11.1 ± 0.2 | 6.7 ± 0.1 | 7.8 ± 0.1 | 9.7 ± 0.1 | 8.7 ± 0.2 | 10.2 |
| 10′ | Corosolic acid | 2.3 ± 0.1 | 9.8 ± 0.2 | 5.2 ± 0.1 | 11.9 ± 0.2 | 8.8 ± 0.2 | 35.7 ± 0.5 | 2.8 ± 0.1 | 5.2 ± 0.1 | 28.4 ± 0.5 | 5.2 ± 0.1 | 11.5 |
| 11′ | 1-Hydroxy-3-oxours-12-en-28-oic acid/isomer | N.D. | 0.9 ± 0.0 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.1 |
| 12′ | 1-Hydroxy-3-oxours-12-en-28-oic acid/isomer | N.D. | 0.9 ± 0.0 | 1.3 ± 0.0 | 2.1 ± 0.0 | N.D. | 0.9 ± 0.0 | N.D. | N.D. | 0.3 ± 0.0 | N.D. | 0.5 |
| 13′ | Betulinic acid | 8.1 ± 0.1 | 1.4 ± 0.0 | 1.2 ± 0.0 | 2.3 ± 0.0 | 2.6 ± 0.0 | 6.2 ± 0.1 | 13.0 ± 0.1 | 1.8 ± 0.0 | 0.6 ± 0.0 | 1.6 ± 0.0 | 3.9 |
| 14′ | Oleanolic acid | 38.5 ± 0.6 | 37.1 ± 0.4 | 35.7 ± 0.4 | 37.2 ± 0.5 | 38.5 ± 0.4 | 49.9 ± 0.6 | 49.0 ± 0.7 | 37.8 ± 0.6 | 13.5 ± 0.3 | 39.6 ± 0.5 | 37.7 |
| 15′ | Ursolic acid | 113.1 ± 1.7 | 112.8 ± 1.1 | 149.7 ± 1.6 | 146.5 ± 1.8 | 129.3 ± 1.3 | 257.1 ± 2.3 | 97.1 ± 1.98 | 125.5 ± 1.8 | 66.7 ± 1.1 | 94.9 ± 1.2 | 129.3 |
| 16′ | 687 m/z [M − H]- | 18.9 ± 0.3 | 20.1 ± 0.4 | 16.1 ± 0.2 | 19.0 ± 0.2 | 11.0 ± 0.2 | 9.8 ± 0.2 | 14.2 ± 0.2 | 19.8 ± 0.2 | 28.0 ± 0.5 | 49.3 ± 0.6 | 20.6 |
| Total triterpenoids | 305.7 ± 1.12 | 367.5 ± 1.4 | 260.3 ± 1.1 | 297.4 ± 1.4 | 274.6 ± 2.6 | 403.1 ± 2.2 | 277.5 ± 3.4 | 274.3 ± 3.2 | 193.0 ± 1.2 | 333.3 ± 2.6 | 298.7 | |
| Pear Variety | Antioxidant Capacity (μmol TE/g FW) | Pear Variety | Antioxidant Capacity (μmol TE/g FW) |
|---|---|---|---|
| DSSL | 20.7 ± 0.2 c | KELXL | 10.1 ± 0.2 g |
| YL | 20.6 ± 0.2 c | XJSL | 22.5 ± 0.3 b |
| CG | 12.4 ± 0.2 f | HQL | 30.4 ± 0.3 a |
| FS | 15.6 ± 0.1 e | SJL | 19.9 ± 0.2 d |
| NGL | 22.2 ± 0.2 b | mean | 19.7 |
| XSL | 22.4 ± 0.2 b | C.V. (%) | 29.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. https://doi.org/10.3390/molecules24010159
Sun L, Tao S, Zhang S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules. 2019; 24(1):159. https://doi.org/10.3390/molecules24010159
Chicago/Turabian StyleSun, Liqiong, Shutian Tao, and Shaoling Zhang. 2019. "Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS" Molecules 24, no. 1: 159. https://doi.org/10.3390/molecules24010159
APA StyleSun, L., Tao, S., & Zhang, S. (2019). Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules, 24(1), 159. https://doi.org/10.3390/molecules24010159