An Unusual Benzoisoquinoline-9-one Derivative and Other Related Compounds with Antiproliferative Activity from Hawaiian Endophytic Fungus Peyronellaea sp. FT431
Abstract
:1. Introduction
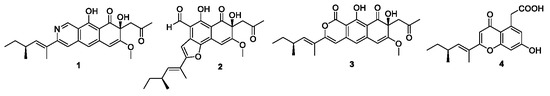
2. Results and Discussions
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation and Identification of Fungal Strain
3.3. Cultivation
3.4. Isolation of Compounds 1–5
3.5. Charaterization of Compounds 1–4
3.6. Anti-Proliferative Activity
3.7. DP4+ Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khaarwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stiele, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; McMillin, D.W.; Tamayo, G.; Delmore, J.; Mitsiades, C.S.; Clardy, J. Inhibition of tumor cells interacting with stromal cells by xanthones isolated from a Costa Rican Penicillium sp. J. Nat. Prod. 2012, 75, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Cryan, L.; Habeshian, K.A.; Murillo, C.; Tamayo-Castillo, G.; Rogers, M.S.; Clardy, J. Phenolic compounds as antiangiogenic CMG2 inhibitors from Costa Rican endophytic fungi. Bioorg. Med. Chem. Lett. 2012, 22, 5885–5888. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Clardy, J. New naphthoquinones and a new δ-lactone produced by endophytic fungi from Costa Rica. Tetrahedron Lett. 2011, 52, 2206–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Ross, L.; Tamayo, G.; Clardy, J. Asterogynins: Secondary metabolites from a Costa Rican endophytic fungus. Org. Lett. 2010, 12, 4661–4663. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Yang, B.J.; Fenstemacher, R.; Turkson, J.; Cao, S. Lycopodiellactone, an unusual δ-lactone-isochromanone from a Hawaiian plant-associated fungus Paraphaeosphaeria neglecta FT462. Tetrahedron Lett. 2015, 56, 1724–1727. [Google Scholar] [CrossRef]
- Li, C.S.; Ding, Y.; Yang, B.J.; Miklossy, G.; Yin, H.Q.; Walker, L.A.; Turkson, J.; Cao, S. A New Metabolite with a Unique 4-Pyranone-γ-Lactam-1,4-Thiazine Moiety from a Hawaiian-Plant Associated Fungus. Org. Lett. 2015, 17, 3556–3559. [Google Scholar] [CrossRef]
- Li, C.S.; Ding, Y.; Yang, B.J.; Hoffman, N.; Yin, H.Q.; Mahmud, T.; Turkson, J.; Cao, S. Eremophilane sesquiterpenes from Hawaiian endophytic fungus Chaetoconis sp. FT087. Phytochemistry 2016, 126, 41–46. [Google Scholar] [CrossRef]
- Li, C.S.; Ren, G.; Yang, B.J.; Miklossy, G.; Turkson, J.; Fei, P.; Ding, Y.; Walker, L.A.; Cao, S. Meroterpenoids with Antiproliferative Activity from a Hawaiian-Plant Associated Fungus Peyronellaea coffeae-arabicae FT238. Org. Lett. 2016, 18, 2335–2338. [Google Scholar] [CrossRef]
- Fei-Zhang, D.J.; Li, C.S.; Cao, S. Hawaii natural compounds are promising to reduce ovarian cancer deaths. Cancer Biol. Ther. 2016, 17, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Yang, B.J.; Turkson, J.; Cao, S. Anti-proliferative ambuic acid derivatives from Hawaiian endophytic fungus Pestalotiopsis sp. FT172. Phytochemistry 2017, 140, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Sarotti, A.M.; Turkson, J.; Cao, S. Verbenanone, an octahydro-5H-chromen-5-one from a Hawaiian-plant associated fungus FT431. Tetrahedron Lett. 2017, 58, 2290–2293. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, C.S.; Sarotti, A.M.; Turkson, J.; Cao, S. Sphaerialactonam, a γ-lactam–isochromanone from the Hawaiian endophytic fungus Paraphaeosphaeria sp. FT462. Tetrahedron Lett. 2017, 58, 1330–1333. [Google Scholar] [CrossRef]
- Li, C.S.; Sarotti, A.M.; Yang, B.J.; Turkson, J.; Cao, S. A New N-methoxypyridone from the Co-Cultivation of Hawaiian Endophytic Fungi Camporesia sambuci FT1061 and Epicoccum sorghinum FT1062. Molecules 2017, 22, 1166. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Sarotti, A.M.; Huang, P.; Dang, U.T.; Hurdle, J.G.; Kondratyuk, T.P.; Pezzuto, J.M.; Turkson, J.; Cao, S. NF-κB inhibitors, unique γ-pyranol-γ-lactams with sulfide and sulfoxide moieties from Hawaiian plant Lycopodiella cernua derived fungus Paraphaeosphaeria neglecta FT462. Sci. Rep. 2017, 7, 10424. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Sarotti, A.M.; Yoshida, W.; Cao, S. Two new polyketides from Hawaiian endophytic fungus Pestalotiopsis sp. FT172. Tetrehedron Lett. 2018, 58, 42–45. [Google Scholar] [CrossRef]
- Li, C.S.; Hu, Z.; Liu, Q.; Wu, X.; Cao, S. Two new tricycloalternarenes from Hawaiian endophytic fungus Didymella sp. FT433. Tetrahedron Lett. 2018, 59, 3381–3383. [Google Scholar] [CrossRef]
- Guerriero, A.; Amrosio, M.; Cuomo, V.; Pietra, F. A Novel, Degraded Polyketidic Lactone, Leptosphaerolide, and Its Likely Diketone Precursor, Leptosphaerodione. Isolation from Cultures of the Marine Ascomycete Leptosphaeria oraemaris (LINDER). Helv. Chim. Acta. 1991, 74, 1445–1450. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nonaka, G.I.; Nishioka, I. Studies on Rhubarb (Rhei Rhizoma). V. Isolation and Characterization of Chromone and Chromanone Derivatives. Chem. Pharm. Bull. 1984, 32, 3493–3500. [Google Scholar] [CrossRef]
- Grimblat, N.; Sarotti, A.M. Computational Chemistry to the Rescue: Modern Toolboxes for the Assignment of Complex Molecules by GIAO NMR Calculations. Chem. Eur. J. 2016, 22, 12246–12261. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G.; Goodman, J.M. Assigning stereochemistry to single diastereoisomers by GIAO NMR calculation: The DP4 probability. J. Am. Chem. Soc. 2010, 132, 12946–12959. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, M.M.; Suárez, A.G.; Sarotti, A.M. Determination of the Relative Configuration of Terminal and Spiroepoxides by Computational Methods. Advantages of the Inclusion of Unscaled Data. J. Org. Chem. 2017, 82, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, M.M.; Biglione, F.A.; Sortino, M.A.; Sarotti, A.M. General Quantum-Based NMR Method for the Assignment of Absolute Configuration by Single or Double Derivatization: Scope and Limitations. J. Org. Chem. 2018, 83, 11839–11849. [Google Scholar] [CrossRef] [PubMed]
- Parisot, D.; Devys, M.; Barbier, M. 5-Deoxybostrycoidin, a New Metabolite Produced by the Fungus Nectria haematococca (Berk, and Br.) Wr. Z. Naturforsch. 1989, 44b, 1473–1474. [Google Scholar] [CrossRef]
- Arsenault, G.P. The structure of bostrycoidin, a β-aza-anthraquinone from Fusariumsolani D2 purple. Tetrahedron Lett. 1965, 45, 4033–4037. [Google Scholar] [CrossRef]
- Graefe, U.; Ihn, W.; Tresselt, D.; Miosga, N.; Kaden, U.; Schlegel, B.; Bormann, E.J.; Sedmera, P.; Novak, J. Tolypocladin—A new metal-chelating 2-aza-anthraquinone from Tolypocladium inflatum. Biol. Met. 1990, 3, 39–44. [Google Scholar] [CrossRef]
- Albinati, A.; Arnone, A.; Assante, G.; Meille, S.V.; Nasini, G. Isoflavans from Millettia racemosa. Phytochemistry 1989, 28, 923–927. [Google Scholar] [CrossRef]
- Coval, S.J.; Hradil, C.M.; Lu, H.S.M.; Clardy, J.; Satouri, S.; Strobel, G.A. Pyrenoline-A and -B, two new phytotoxins from Pyrenophora teres. Tetrahedron Lett. 1990, 31, 2117–2120. [Google Scholar] [CrossRef]
- Yue, P.; Zhang, X.; Paladino, D.; Sengupta, B.; Ahmad, S.; Holloway, R.W.; Ingersoll, S.B.; Turkson, J. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene 2012, 31, 2309–2322. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tapia, F.; Brotherton-Pleiss, C.; Yue, P.; Murakami, H.; Costa Araujo, A.C.; Reis Dos Santos, B.; Ichinotsubo, E.; Rabkin, A.; Shah, R.; Lantz, M.; et al. Linker Variation and Structure-Activity Relationship Analyses of Carboxylic Acid-based Small Molecule STAT3 Inhibitors. ACS Med. Chem. Lett. 2018, 9, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Lopez-Tapia, F.; Paladino, D.; Li, Y.; Chen, C.-H.; Namanja, A.T.; Hilliard, T.; Chen, Y.; Tius, M.; Turkson, J. Hydroxamic acid and benzoic acid-based Stat3 inhibitors suppress human glioma and breast cancer phenotypes in vitro and in vivo. Cancer Res. 2016, 76, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, P.; Page, B.D.; Li, T.; Zhao, W.; Namanja, A.T.; Paladino, D.; Zhao, J.; Chen, Y.; Gunning, P.T.; et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc. Natl. Acad. Sci. USA 2012, 109, 9623–9628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, C.01 ed. Gaussian, Inc.: Wallingford, CT, USA, 2009.
- MacroModel Schrodinger Release 2018-3, Schrodinger LLC: New York, NY, USA, 2018.
- Ditchfield, R. Molecular Orbital Theory of Magnetic Shielding and Magnetic Susceptibility. J. Chem. Phys. 1972, 56, 5688–5691. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for NMR chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- McMichael Rohlfing, C.; Allen, L.C.; Ditchfield, R. Proton and carbon-13 chemical shifts: Comparison between theory and experiment. Chem. Phys. 1984, 87, 9–15. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH, J (Hz) a | δC b | δH, J (Hz) a | δC b | |
| 1 | 9.43, s | 148.4 | 10.52, s | 187.6 |
| 3 | 158.5 | 166.87 | ||
| 4 | 7.61, s | 115.0 | 7.42, s | 104.2 |
| 4a | 143.3 | 112.2 | ||
| 5 | 7.05, s | 114.7 | 144.2 | |
| 5a | 139.0 | 136.3 | ||
| 6 | 6.00, s | 98.9 | 6.33, s | 90.7 |
| 7 | 161.6 | 166.92 | ||
| 8 | 72.8 | 73.2 | ||
| 9 | 202.8 | 202.6 | ||
| 9a | 107.7 | 106.4 | ||
| 10 | 164.7 | |||
| 10a | 117.9 | 130.0 | ||
| 11 | 133.3 | 124.6 | ||
| 12 | 6.70, d, 10 | 140.1 | 141.0 | |
| 13 | 2.60, m | 35.7 | 2.64, m | 35.5 |
| 14 | 1.49, m;1.41, m | 31.0 | 1.49, m;1.42, m | 30.8 |
| 15 | 0.92, t, 7.4 | 12.4 | 0.92, t, 7.4 | 12.4 |
| 16 | 2.16, d, 1.3 | 14.5 | 1.45, d, 1.4 | 13.6 |
| 17 | 1.07, d, 6.7 | 20.8 | 1.10, d, 6.6 | 20.5 |
| 1′ | 3.52, s | 50.6 | 3.59, d, 5.5 | 51.3 |
| 2′ | 205.7 | 206.5 | ||
| 3′ | 2.09, s | 29.6 | 2.13, s | 29.8 |
| 7-MeO | 3.79, s | 56.2 | 3.91, s | 56.9 |
| No. | 3 a | 4 b | ||
|---|---|---|---|---|
| δH, J (Hz) | δC | δH, J (Hz) | δC | |
| 1 | 164.8 | 181.6 | ||
| 2 | 6.16, s | 107.4 | ||
| 3 | 158.0 | 164.3 | ||
| 4 | 6.56 | 101.6 | 160.3 | |
| 4a | ||||
| 5 | 6.77, s | 114.1 | 6.78, d, 2.0 | 102.1 |
| 5a | ||||
| 6 | 5.89, s | 97.1 | 163.1 | |
| 7 | 163.1 | 6.69, d, 2.6 | 118.5 | |
| 8 | 72.1 | 140.2 | ||
| 9 | 199.9 | 115.4 | ||
| 9a | 109.1 | |||
| 10 | 159.1 | 126.8 | ||
| 10a | 104.4 | |||
| 11 | 125.6 | 142.8 | ||
| 12 | 6.34, d, 9.7 | 140.3 | 2.59, m | 35.8 |
| 13 | 2.58, m | 34.7 | 1.52, m; 1.42, m | 30.6 |
| 14 | 1.48, m; 1.41, m | 29.8 | 0.92, t, 7.6 | 12.0 |
| 15 | 0.89, t, 7.4 | 11.4 | 1.97, s | 12.7 |
| 16 | 1.98, s | 11.9 | 1.08, d, 6.7 | 20.3 |
| 17 | 1.05, d, 6.6 | 19.6 | ||
| 1′ | 3.46, br.d, 5.9 | 50.6 | 4.11, s | 42.8 |
| 2′ | 205.7 | 176.9 | ||
| 3′ | 2.10, s | 29.0 | ||
| 7-MeO | 3.79, s | 56.2 | 107.4 | |
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| A2780S | A2780CisR | TK-10 | |
| 1 | 24.1 ± 0.8 | 28.3 ± 7.2 | 29.2 ± 2.9 |
| 2 | 21.5 ± 0.3 | 27.2 ± 1.3 | 22.7 ± 1.3 |
| 5 | 7.1 ± 0.8 | 6.7 ± 1.2 | 8.5 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Sarotti, A.M.; Wu, X.; Yang, B.; Turkson, J.; Chen, Y.; Liu, Q.; Cao, S. An Unusual Benzoisoquinoline-9-one Derivative and Other Related Compounds with Antiproliferative Activity from Hawaiian Endophytic Fungus Peyronellaea sp. FT431. Molecules 2019, 24, 196. https://doi.org/10.3390/molecules24010196
Li C, Sarotti AM, Wu X, Yang B, Turkson J, Chen Y, Liu Q, Cao S. An Unusual Benzoisoquinoline-9-one Derivative and Other Related Compounds with Antiproliferative Activity from Hawaiian Endophytic Fungus Peyronellaea sp. FT431. Molecules. 2019; 24(1):196. https://doi.org/10.3390/molecules24010196
Chicago/Turabian StyleLi, Chunshun, Ariel M. Sarotti, Xiaohua Wu, Baojun Yang, James Turkson, Yongfei Chen, Qingsong Liu, and Shugeng Cao. 2019. "An Unusual Benzoisoquinoline-9-one Derivative and Other Related Compounds with Antiproliferative Activity from Hawaiian Endophytic Fungus Peyronellaea sp. FT431" Molecules 24, no. 1: 196. https://doi.org/10.3390/molecules24010196
APA StyleLi, C., Sarotti, A. M., Wu, X., Yang, B., Turkson, J., Chen, Y., Liu, Q., & Cao, S. (2019). An Unusual Benzoisoquinoline-9-one Derivative and Other Related Compounds with Antiproliferative Activity from Hawaiian Endophytic Fungus Peyronellaea sp. FT431. Molecules, 24(1), 196. https://doi.org/10.3390/molecules24010196