Use of Confectionery Waste in Biogas Production by the Anaerobic Digestion Process
Abstract
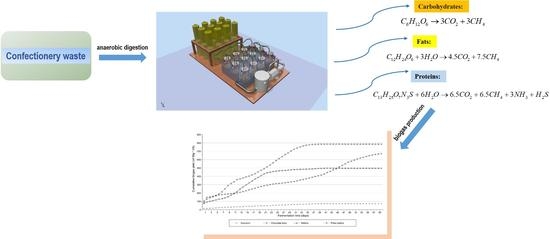
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Calculation of Cumulative Biogas and Methane
3. Results and Discussion
3.1. Characterisation of Substrates and Biodegradation
3.2. Process Stability
3.3. Biogas Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AD | anaerobic digestion |
| AcoD | anaerobic co-digestion |
| Cond. | conductivity (mS cm−1) |
| TS | total solids (wt %) |
| VS | volatile solids (wt %TS) |
| TOC | total organic carbon (wt %TS) |
| TKN | total Kjeldahl nitrogen (wt %TS; g kg−1TS) |
| TAN | total ammonium nitrogen (wt %TS) |
| Ptotal | total phosphorus (wt %TS) |
| COD | chemical oxygen demand (mg L−1) |
| VFA | volatile fatty acids (mg acetic acid L−1) [flüchtigen organischen säuren, FOS—in German] |
| TA | total alkalinity (mg CaCO3 L−1) [total alkalischen carbonaten, TAC—in German] |
| VFA/TA ratio | volatile fatty acids-to-total alkalinity ratio. |
References
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 30, 3–16. [Google Scholar] [CrossRef]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Dach, J.; Czekała, W.; Boniecki, P.; Lewicki, A.; Piechota, T. Specialised internet tool for biogas plant modelling and marked analyzing. Adv. Mat. Res. 2014, 909, 305–310. [Google Scholar]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Pfaltzgraff, L.A.; De bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Kader, F.; Baky, A.H.; Khan, M.N.H.; Chowdhury, H.A. Production of biogas by anaerobic digestion of food waste and process simulation. Am. J. Mech. Eng. 2015, 3, 79–83. [Google Scholar]
- Razaviarani, V.; Buchanan, I.D.; Malik, S.; Katalambula, H. Pilot-scale anaerobic co-digestion of municipal wastewater sludge with restaurant grease trap waste. J. Environ. Manag. 2013, 123, 26–33. [Google Scholar] [CrossRef]
- Montanés, R.; Pérez, M.; Solera, R. Anaerobic mesophilic co-digestion of sewage sludge and sugar beet pulp lixiviation in batch reactors: Effect of pH control. Chem. Eng. J. 2014, 255, 492–499. [Google Scholar] [CrossRef]
- Silvestre, G.; Illa, J.; Fernández, B.; Bonmatí, A. Thermophilic anaerobic co-digestion of sewage sludge with grease waste: Effect of long chain fatty acids in the methane yield and its dewatering properties. Appl. Energy 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Lafitte-Trouqué, S.; Forster, C.F. Dual anaerobic co-digestion of sewage sludge and confectionery waste. Bioresour. Technol. 2000, 71, 77–82. [Google Scholar] [CrossRef]
- Rusín, J.; Kašáková, K.; Chamrádová, K. Anaerobic digestion of waste wafer material from the confectionery production. Energy 2015, 85, 194–199. [Google Scholar] [CrossRef]
- Pilarska, A.A. Anaerobic co-digestion of waste wafers from the confectionery production with sewage sludge. Polish J. Environ. Stud. 2018, 27, 237–245. [Google Scholar] [CrossRef]
- Lunghi, P.; Burzacca, R. Energy recovery from industrial waste of a confectionery plant by means of BIGFC plant. Energy 2004, 29, 2601–2617. [Google Scholar] [CrossRef]
- Leite, A.F.; Janke, L.; Lv, Z.; Harms, H.; Richnow, H.H.; Nikolausz, M. Improved Monitoring of semi-continuous anaerobic digestion of sugarcane waste: Effects of increasing organic loading rate on methanogenic community dynamics. Int. J. Mol. Sci. 2015, 16, 23210–23226. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Strik, D.P.B.T.B.; Domnanovich, A.M.; Holubar, P. A pH-based control of ammonia in biogas during anaerobic digestion of artificial pig manure and maize silage. Process Biochem. 2006, 41, 1235–1238. [Google Scholar] [CrossRef]
- Callaghan, F.J.; Wase, D.A.J.; Thayanithy, K.; Forster, C.F. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure. Biomass Bioenergy 2002, 27, 71–77. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zarkadas, I.; Dontis, G.; Pilidis, G.; Sarigiannis, D.A. Exploring the potential of fur farming wastes and by products as substrates to anaerobic digestion process. Renew. Energy 2016, 96, 1063–1070. [Google Scholar] [CrossRef]
- Fermentation of Organic Materials Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Norm VDI 4630; German Engineers Club: Düsseldorf, Germany, 2006.
- German Institute for Standardization. Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Norm DIN 38 414-S8; German Institute for Standardization: Berlin, Germany, 1985. [Google Scholar]
- Dach, J.; Koszela, K.; Boniecki, P.; Zaborowicz, M.; Lewicki, A.; Czekała, W.; Skwarcz, J.; Qiao, W.; Piekarska-Boniecka, H.; Białobrzewski, I. The use of neural modelling to estimate the methane production from slurry fermentation processes. Renew. Sustain. Energy Rev. 2016, 56, 603–610. [Google Scholar] [CrossRef]
- AOAC International. Association of Analytical Chemists Official Method 920.87. Protein (Total) in Flour. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- AOAC International. Association of Analytical Chemists Official Method 920.85. Fat (Crude) or Ether Extract of Flour. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- AOAC International. Association of Analytical Chemists Official Method 962.09. Fiber (crude) in animal feed and pet food. In The Official Methods of Analysis of AOAC International, 16th ed.; Cunniff, P., Ed.; AOAC: Rockville, MD, USA, 1995. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Witaszek, K.; Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Kolasiński, M.; Szwarc-Rzepka, K. Treatment of dairy waste by anaerobic digestion with sewage sludge. Ecol. Chem. Eng. S 2016, 23, 99–115. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Ryniecki, A.; Tomaszyk, K.; Dach, J.; Wolna-Maruwka, A. Utilization of vegetable dumplings waste from industrial production by anaerobic digestion. Int. Agrophys. 2017, 31, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources, 2nd ed.; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Uellendahl, H.; Wang, G.; Mřller, H.B.; Jřrgensen, U.; Skiadas, V.; Gaval, H.M.; Ahring, B.K. Energy balance and cost-benefit analysis of biogas production from perennial energy crops pretreated by wet oxidation. Water Sci. Technol. 1998, 58, 1841–1847. [Google Scholar] [CrossRef]
- Pilarski, K.; Pilarska, A.A.; Witaszek, K.; Dworecki, Z.; Żelaziński, T.; Ekielski, A.; Makowska, A.; Michniewicz, J. The impact of extrusion on the biogas and biomethane yield of plant substrates. J. Ecol. Eng. 2016, 17, 264–272. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Clayden, J.; Greeves, N.; Warren, S.; Wothers, P.D. Organic Chemistry; Oxford University Press Inc.: New York, NY, USA, 2001. [Google Scholar]
- Wan, C.; Zhou, Q.; Fu, G.; Yebo, L. Semi-continuous anaerobic co-digestion of thickened waste activated sludge and fat, oil and grease. Waste Manag. 2011, 31, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lang, Q.; Fang, M.; Li, X.; Bah, H.; Dong, H.; Dong, R. Combined effect of crude fat content and initial substrate concentration on batch anaerobic digestion characteristics of food waste. Bioresour. Technol. 2017, 232, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Cerin, S.; Nowakowski, K.; Dach, J.; Pilarski, K.; Boniecki, P.; Przybyl, J.; Lewicki, A. Possibilities of neural image analysis implementation in monitoring of microalgae production as a substrate for biogas plant. In Proceedings of the SPIE 4th International Conference on Digital Image Processing, Kuala Lumpur, Malaysia, 7–8 April 2012; Volume 8334. [Google Scholar]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of desugared molasses with cow manure; focusing on sodium and potassium inhibition. Bioresour. Technol. 2011, 102, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.; Muñoz, N.; Fernández, J.; Rico, J.L. High-load anaerobic co-digestion of cheese whey and liquid fraction of dairy manure in a one-stage UASB process: Limits in co-substrates ratio and organic loading rate. Chem. Eng. J. 2015, 262, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, C.; Liu, X.; Ma, H.; Wu, J.; Zuo, J.; Wang, K. A new method of two-phase anaerobic digestion for fruit and vegetable waste treatment. Bioresour. Technol. 2016, 211, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K. Kraft lignin grafted with polyvinylpyrrolidone as a novel microbial carrier in biogas production. Energies 2018, 11, 3246. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Indicator | Unit | Chocolate Bars (CB) | Wafers (W) | Filled Wafers (FW) | Inoculum | LSD0.05 |
|---|---|---|---|---|---|---|
| General | ||||||
| pH | – | 6.62 (0.10) | 7.84 (0.33) | 7.02 (0.27) | 7.68 (0.04) | 0.46 |
| Cond. | mS cm−1 | 1.21 (0.04) | 1.76 (0.03) | 1.85 (0.06) | 26.50 (0.62) | 0.66 |
| TS | wt % | 94.80 (0.81) | 97.69 (0.28) | 96.77 (0.05) | 3.18 (0.02) | 0.31 |
| VS | wt %TS | 98.31 (1.02) | 98.44 (0.05) | 98.86 (0.03) | 70.43 (0.22) | 1.10 |
| TOC | wt %TS | 45.2 (0.53) | 41.6 (0.36) | 43.9 (0.40) | 32.2 (0.36) | 0.87 |
| TKN | wt %TS | 0.86 (0.01) | 1.25 (0.02) | 0.98 (0.03) | 2.91 (0.02) | 0.048 |
| TOC/TKN ratio | – | 52.6 (0.65) | 33.3 (0.70) | 44.8 (0.26) | 11.0 (0.55) | 1.21 |
| TKN a | mg kg−1 | 4.41 (0.04) | 13.78 (0.03) | 9.48 (0.04) | ND b | 3.21 |
| TAN | wt %TS | 0.26 (0.01) | 0.23 (0.04) | 0.33 (0.02) | 2.6 (0.10) | 0.12 |
| Ptotal | wt %TS | 0.53 (0.04) | 0.45 (0.04) | 0.62 (0.03) | 0.30 (0.03) | 0.07 |
| COD | mg L−1 | 1875 (6.55) | 1128 (2.64) | 1410 (4.58) | 1643 (3.60) | 87.11 |
| Light metal ions | ||||||
| K | mg kg−1 | 74.5 (0.43) | 55.3 (0.17) | 51.5 (0.36) | 65.1 (0.43) | 5.77 |
| Na | mg kg−1 | 151.1 (0.85) | 135.3 (0.26) | 168.2 (0.30) | 35.9 (0.17) | 11.01 |
| Mg | mg kg−1 | 35.0 (0.45) | 41.1 (0.91) | 32.3 (0.61) | 10.3 (0.36) | 1.31 |
| Ca | mg kg−1 | 71.2 (2.16) | 56.5 (1.86) | 67.0 (1.96) | 30.8 (0.36) | 5.66 |
| Biochemical composition | ||||||
| Crude protein a | g kg−1 | 27.6 (0.45) | 84.3 (0.36) | 59.3 (0.36) | ND | 35.27 |
| Crude fat | g kg−1 | 212.2 (0.72) | 45.0 (0.36) | 282.5 (0.72) | ND | 46.78 |
| Crude fiber | g kg−1 | 14.5 (0.26) | 51.9 (0.52) | 32.0 (0.55) | ND | 15.97 |
| Carbohydrate | ||||||
| Sucrose | g kg−1 | 489.8 (1.60) | 11.2 (0.17) | 419.4 (0.65) | ND | 45.21 |
| Starch | g kg−1 | 651.9 (0.17) | 757.7 (0.85) | 591.1 (1.01) | ND | 11.47 |
| Batch | Substrate (g) | Inoculum (g) | pH | TOC/TKN Ratio | TS (%) |
|---|---|---|---|---|---|
| CB/inoculum | 50 | 1000 | 6.82 (0.02) | 15 (0.70) | 7.54 (0.10) |
| W/inoculum | 50 | 1000 | 6.94 (0.05) | 17 (0.26) | 7.68 (0.10) |
| FW/inoculum | 50 | 1000 | 7.01 (0.11) | 16 (0.26) | 7.63 (0.05) |
| Parameter | Method and Standard |
|---|---|
| pH | Potentiometric analysis (Elmetron CP-215, Elmetron, Zabrze, Poland); PN-EN 12176:2004, EN 15933:2012 |
| TS | Gravimetric analysis, 105 °C (dryer Zalmed SML 30, Zalmed, Łomianki, Poland); PN-EN 12880:2004, EN 15934:2012 |
| VS | Gravimetric analysis, 550 °C (furnace MS Spectrum PAF 110/6, Protherm Furnaces, Ankara, Turkey); PN-EN 12879:2004, EN 15935:2012 |
| Cond. | Conductivity analysis (Elmetron CP-215, Elmetron, Zabrze, Poland); PN-EN 27888:1999. |
| TOC | Combustion (900 °C), CO2 determination (Infrared Spectrometry, O-I analytical analyser, SRA Instruments, Lyon, France); PB/PFO-37, EN 15936:2012 |
| TKN | Titration, Kjeldahl method, 0.1n HCl, Tashiro’s indicator; PN-EN 13342, EN 15104:2011 |
| TAN | Distillation and titration an method, 0.1n HCl, Tashiro’s indicator; PN-ISO 5664, ISO 5664 |
| Ptotal | Mineralization of phosphorus compounds with nitric acid (microwave furnace, Milestone, Hanon Instruments, Jinan, China), spectrophotometric analysis (Varian Cary 50, Varian Medical System, Palo Alto, CA, USA); PB/PFO-11, EN 14672:2005 |
| VFA/TA ratio * | Titration with 0.05 M H2SO4 to two end values (pH 5.0 and 4.4) |
| COD | Titration, dichromate method (potassium dichromate, concentrated sulphuric acid, silver sulfate as catalyst); PN-ISO 6060-2006 |
| Light metal ions | Inductively coupled plasma optical emission spectrometry (ICP-OES, JY 2000 2 ICP-OES Spectrometer, Hitachi, Tokyo, Japan); PN-EN ISO 11885:2009 |
| Crude proteins | Calculated from TKN using a conversion factor of 6.25 for crude proteins; AOAC 920.87 [28] |
| Crude fats | Soxhlet method; extracted with hexane by using an automatic extractor Soxhlet model B-811 BUCHI, (Büchi Labortechnik AG, Flawil, Switzerland); AOAC 920.85 [29]. |
| Crude fibre | Chemical method (digestion in 0.25N H2SO4 and then 0.25N NaOH) AOAC 962.09 [30] |
| Carbohydrates | Phenol–sulphuric acid methods [31] |
| Substrates | Proteins | Fats | Fiber | Sucrose | Starch |
|---|---|---|---|---|---|
| Chocolate bars | 0.97 * | 0.80 | 0.98 * | −0.72 | −0.17 |
| Wafers | 0.78 | −0.78 | 0.72 | 0.96 * | 0.79 |
| Filled wafers | 0.97 * | 0.56 | −0.63 | −0.75 | 0.85 |
| Batch | Biogas | Methane | CH4 | Theoretical BMP | ||
|---|---|---|---|---|---|---|
| (m3 Mg−1 FM) | (m3 Mg−1 VS) | (m3 Mg−1 FM) | (m3 Mg−1 VS) | (%) | (m3 Mg−1 VS) | |
| Inoculum | 1.61 (0.16) | 71.91 (7.12) | 0.51 (0.07) | 22.94 (5.17) | 31.9 (6.69) | – |
| CB/inoculum | 627.67 (5.37) | 673.48 (19.97) | 379.74 (10.13) | 407.46 (5.90) | 60.5 (1.66) | 572.10 |
| W/inoculum | 479.77 (27.13) | 496.78 (57.85) | 306.55 (13.71) | 317.42 (8.88) | 63.9 (5.85) | 349.14 |
| FW/inoculum | 749.19 (9.61) | 684.79 (18.59) | 483.35 (12.92) | 506.32 (5.32) | 73.9 (8.72) | 580.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of Confectionery Waste in Biogas Production by the Anaerobic Digestion Process. Molecules 2019, 24, 37. https://doi.org/10.3390/molecules24010037
Pilarska AA, Pilarski K, Wolna-Maruwka A, Boniecki P, Zaborowicz M. Use of Confectionery Waste in Biogas Production by the Anaerobic Digestion Process. Molecules. 2019; 24(1):37. https://doi.org/10.3390/molecules24010037
Chicago/Turabian StylePilarska, Agnieszka A., Krzysztof Pilarski, Agnieszka Wolna-Maruwka, Piotr Boniecki, and Maciej Zaborowicz. 2019. "Use of Confectionery Waste in Biogas Production by the Anaerobic Digestion Process" Molecules 24, no. 1: 37. https://doi.org/10.3390/molecules24010037
APA StylePilarska, A. A., Pilarski, K., Wolna-Maruwka, A., Boniecki, P., & Zaborowicz, M. (2019). Use of Confectionery Waste in Biogas Production by the Anaerobic Digestion Process. Molecules, 24(1), 37. https://doi.org/10.3390/molecules24010037