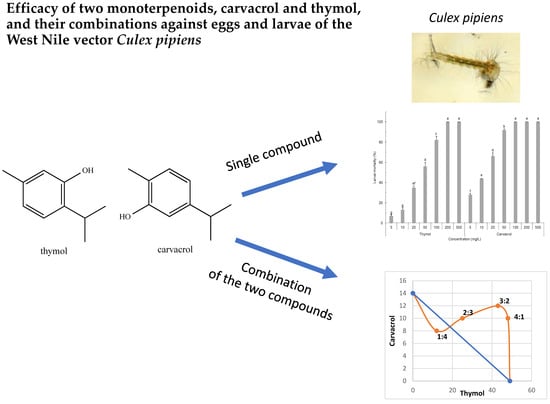
Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex pipiens
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Ovicidal and Larvicidal Efficacy
3.2. Ovicidal and Larvicidal Efficacy of the Blend Containing Carvacrol and Thymol
4. Materials and Methods
4.1. Chemicals
4.2. Ovicidal and Larvicidal Assays
4.3. Combinational Bioassays
4.4. Statistical Analysis
5. Conclusions and Outlooks for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benelli, G.; Beier, J. Current vector control challenges in the fight against malaria. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Badawy, M.E.; Taktak, N.E.; Awad, O.M.; Elfiki, S.A.; Abou El-Ela, N.E. Preparation of ecofriendly formulations containing biologically active monoterpenes with their fumigant and residual toxicities against adults of Culex pipiens. J. Trop. Med. 2016, 2016, 8540830. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Best practices for preventing vector-borne diseases in dogs and humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef]
- Fernandes, J.N.; Moise, I.K.; Maranto, G.L.; Beier, J.C. Revamping mosquito-borne disease control to tackle future threats. Trends Parasitol. 2018, 34, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Romano, D. Mosquito vectors of Zika virus. Entomol. Gen. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and Zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- Wilke, A.B.; Beier, J.C.; Benelli, G. Transgenic mosquitoes–fact or fiction? Trends Parasitol. 2018, 34, 456–465. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. A renaissance for botanical insecticides? Pest. Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant. Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop. Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant. Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Beyond mosquitoes – Essential oil toxicity and repellency against bloodsucking insects. Ind. Crops Prod. 2018, 117, 382–392. [Google Scholar] [CrossRef]
- Benelli, G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: A systematic review. Parasitol. Res. 2015, 114, 3201–3212. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crops Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Barimani, A.; Araghi, A. Carvacrol as a potent natural acaricide against Dermanyssus gallinae. Parasitol. Res. 2015, 114, 3801–3806. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Youssefi, M.R.; Esfandiari, A.; Benelli, G. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil against the West Nile and filariasis vector Culex pipiens (Diptera: Culicidae). Res. Vet. Sci. 2017, 114, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Kavallieratos, N.G.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Benelli, G. Rationale for developing novel mosquito larvicides based on isofuranodiene microemulsions. J. Pest. Sci. 2019, 92, 909–921. [Google Scholar] [CrossRef]
- Koliopoulos, G.; Pitarokili, D.; Kioulos, E.; Michaelakis, A.; Tzakou, O. Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol. Res. 2010, 107, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, F.; Youssefi, M.R.; Tabari, M.A. Combination of carvacrol and thymol against the poultry red mite (Dermanyssus gallinae). Parasitol. Res. 2016, 115, 4239–4243. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; Chichester: John Wiley & Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Lupidi, G.; Maggi, F. Comparison of chemical composition and antioxidant activities of two winter savory subspecies (Satureja montana subsp. variegata and Satureja montana subsp. montana) cultivated in Northern Italy. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E. The chemical composition of Thymus oils: A review of the literature 1960–1989. J. Essent. Oil Res. 1991, 3, 61–82. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Maggi, F.; Nkuimi Wandjou, J.G.; N’ Guessan Bra, Y.F.; Koné-Bamba, D.; Sagratini, G.; Vittori, S.; Caprioli, G. Insecticidal activity of the essential oil and polar extracts from Ocimum gratissimum grown in Ivory Coast: Efficacy on insect pests and vectors and impact on non-target species. Ind. Crops Prod. 2019, 132, 377–385. [Google Scholar] [CrossRef]
- Vitali, L.A.; Beghelli, D.; Nya, P.C.B.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Bruno, M.; Benelli, G. Larvicidal activity of essential oils of five Apiaceae taxa and some of their main constituents against Culex quinquefasciatus. Chem. Biodivers. 2018, 15, e1700382. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.H.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: Larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crops Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Ahn, Y.-J.; Lee, S.-B.; Lee, H.-S.; Kim, G.-H. Insecticidal and acaricidal activity of carvacrol and β-thujaplicine derived from Thujopsis dolabrata var. hondai sawdust. J. Chem. Ecol. 1998, 24, 81–90. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Senra, T.O.S.; Calmon, F.; Zeringóta, V.; Monteiro, C.M.O.; Maturano, R.; da Silva Matos, R.; Daemon, E. Investigation of activity of monoterpenes and phenylpropanoids against immature stages of Amblyomma cajennense and Rhipicephalus sanguineus (Acari: Ixodidae). Parasit Res. 2013, 112, 3471–3476. [Google Scholar] [CrossRef] [PubMed]
- Novato, T.P.L.; Araújo, L.X.; de Monteiro, C.M.O.; Maturano, R.; Senra, T.d.O.S.; da Silva Matos, R.; Gomes, G.A.; de Carvalho, M.G.; Daemon, E. Evaluation of the combined effect of thymol, carvacrol and (E)-cinnamaldehyde on Amblyomma sculptum (Acari: Ixodidae) and Dermacentor nitens (Acari: Ixodidae) larvae. Vet. Parasitol. 2015, 212, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest. Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Upadhyay, S.; Tripathi, A.K. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi. Parasitol. Res. 2009, 105, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism–antagonism effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Abdelgaleil, S.A.; Rabie, R.Y. Insecticidal and synergistic effects of Majorana hortensis essential oil and some of its major constituents. Entomol. Exp. Appl. 2009, 131, 225–232. [Google Scholar] [CrossRef]
- Pavela, R. Acute and synergistic effects of some monoterpenoid essential oil compounds on the house fly (Musca domestica L.). J. Essent. Oil Bear. Plants 2008, 11, 451–459. [Google Scholar] [CrossRef]
- Wu, L.; Huo, X.; Zhou, X.; Zhao, D.; He, W.; Liu, S.; Feng, T.; Wang, C. Acaricidal activity and synergistic effect of thyme oil constituents against carmine spider mite (Tetranychus cinnabarinus (Boisduval)). Molecules 2017, 22, 1873. [Google Scholar] [CrossRef]
- Knio, K.; Usta, J.; Dagher, S.; Zournajian, H.; Kreydiyyeh, S. Larvicidal activity of essential oils extracted from commonly used herbs in Lebanon against the seaside mosquito, Ochlerotatus caspius. Bioresour. Technol. 2008, 99, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.U.; Melo, V.M.M.; Craveiro, A.A.; Machado, M.I.L.; Bantim, M.B.; Rabelo, E.F. Larvicidal activity of the essential oil from Lippia sidoides Cham. against Aedes aegypti Linn. Memórias do Instituto Oswaldo Cruz 2003, 98, 569–571. [Google Scholar] [CrossRef]
- Benelli, G.; Maggi, F.; Canale, A.; Mehlhorn, H. Lyme disease is on the rise—How about tick repellents? A global view. Entomologia Generalis 2019. [Google Scholar] [CrossRef]
- Shaalan, E.A.-S.; Canyon, D.V.; Younes, M.W.F.; Abdel-Wahab, H.; Mansour, A.-H. Synergistic efficacy of botanical blends with and without synthetic insecticides against Aedes aegypti and Culex annulirostris mosquitoes. J. Vector Ecol. 2005, 30, 284–288. [Google Scholar] [PubMed]
- Tong, F.; Bloomquist, J.R. Plant essential oils affect the toxicities of carbaryl and permethrin against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; García, M.; Steinbauer, S.; Setzer, W.N.; Scull, R.; Gille, L.; Monzote, L. Combinations of ascaridole, carvacrol, and caryophyllene oxide against Leishmania. Acta Trop. 2015, 145, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and genotoxic activities of oregano essential oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Araújo, L.X.; Novato, T.P.L.; Zeringota, V.; Maturano, R.; Melo, D.; Da Silva, B.C.; Monteiro, C.M.O. Synergism of thymol, carvacrol and eugenol in larvae of the cattle tick, Rhipicephalus microplus, and brown dog tick, Rhipicephalus sanguineus. Med. Vet. Entomol. 2016, 30, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- López, V.; Cascella, M.; Benelli, G.; Maggi, F.; Gómez-Rincón, C. Green drugs in the fight against Anisakis simplex—larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018, 117, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Azeez, S.; Babu, R.O.; Aykkal, R.; Narayanan, R. Virtual screening and in vitro assay of potential drug like inhibitors from spices against glutathione-S-transferase of filarial nematodes. J. Mol. Model. 2012, 18, 151–163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. 2015. Available online: http://www.who.int/iris/handle/10665/69101 (accessed on 25 March 2019).
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University: London, UK, 1971; pp. 68–78. [Google Scholar]
- Ohrt, C.; Willingmyre, G.D.; Lee, P.; Knirsch, C.; Milhous, W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 2002, 46, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of thymol and carvacrol are available from the authors. |


| Tested Compound | Targeted Instar | LC50 (mg/L) | 95% LCL-UCL | LC90 (mg/L) | 95% LCL-UCL | χ2(df) |
|---|---|---|---|---|---|---|
| Carvacrol | Egg | 7 | 6-8 | 20 | 19-22 | 2.08 (5) n.s. |
| 3rd instar larva | 14 | 11-17 | 44 | 38-52 | 4.23 (5) n.s. | |
| Thymol | Egg | 13 | 12-14 | 27 | 25-31 | 1.56 (5) n.s. |
| 3rd instar larva | 49 | 42-52 | 112 | 99-130 | 2.98 (5) n.s. |
| Thymol:Carvacrol Ratio | LC50 Thymol:Carvacrol in Combination (mg/L) on Eggs | FLC index a on Eggs | LC50 Thymol:Carvacrol in Combination (mg/L) on 3rd Instar Larvae | FLC Index a on 3rd Instar Larvae |
|---|---|---|---|---|
| 4:1 | 10:6 | 1.56 | 48:10 | 1.65 |
| 3:2 | 7.2:6.5 | 1.41 | 43:12 | 2.10 |
| 2:3 | 6:6.2 | 1.28 | 25:10 | 1.19 |
| 1:4 | 3.5:5.5 | 1.00 | 12:8 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssefi, M.R.; Tabari, M.A.; Esfandiari, A.; Kazemi, S.; Moghadamnia, A.A.; Sut, S.; Dall’Acqua, S.; Benelli, G.; Maggi, F. Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex pipiens. Molecules 2019, 24, 1867. https://doi.org/10.3390/molecules24101867
Youssefi MR, Tabari MA, Esfandiari A, Kazemi S, Moghadamnia AA, Sut S, Dall’Acqua S, Benelli G, Maggi F. Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex pipiens. Molecules. 2019; 24(10):1867. https://doi.org/10.3390/molecules24101867
Chicago/Turabian StyleYoussefi, Mohammad Reza, Mohaddeseh Abouhosseini Tabari, Aryan Esfandiari, Sohrab Kazemi, Ali Akbar Moghadamnia, Stefania Sut, Stefano Dall’Acqua, Giovanni Benelli, and Filippo Maggi. 2019. "Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex pipiens" Molecules 24, no. 10: 1867. https://doi.org/10.3390/molecules24101867
APA StyleYoussefi, M. R., Tabari, M. A., Esfandiari, A., Kazemi, S., Moghadamnia, A. A., Sut, S., Dall’Acqua, S., Benelli, G., & Maggi, F. (2019). Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex pipiens. Molecules, 24(10), 1867. https://doi.org/10.3390/molecules24101867