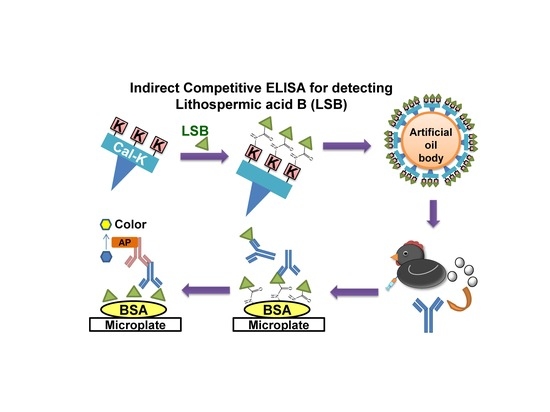
Development of Indirect Competitive ELISA for Lithospermic Acid B of Salvia miltiorrhiza with Its Specific Antibodies Generated via Artificial Oil Bodies
Abstract
:1. Introduction
2. Results
2.1. Conjugation of LSB to Recombinant Caleosin, Cal-K
2.2. Generation of Antibodies via AOBs Constituted with LSB-Cal-K
2.3. Indirect Competitive ELISA for LSB Detection
2.4. Comparison of LSB Contents in Danshen Extracts Detected by HPLC and Indirect Competitive ELISA
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Over Expression and Purification of Recombinant Cal-K
4.3. Conjugation of LSB to Cal-K and BSA
4.4. Preparation of AOBs for Antibody Generation
4.5. Purification of Antibodies from Egg Yolk
4.6. Western Blotting
4.7. Indirect Competitive ELISA
4.8. Detection of LSB Contents in Danshen Extracts by HPLC and the Indirect Competitive ELISA
Author Contributions
Funding
Conflicts of Interest
References
- Wang, B.Q. Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J. Med. Plant Res. 2010, 4, 2813–2820. [Google Scholar]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—a review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Chang, H.M.; Cheng, K.P.; Choang, T.F.; Chow, H.F.; Chui, K.Y.; Hon, P.M.; Tan, F.W.L.; Yang, Y.; Zhong, Z.P. Structure elucidation and total synthesis of new tanshinones isolated from Salvia miltiorrhiza Bunge (Danshen). J. Org. Chem. 1990, 55, 3537–3543. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, H. Anti-Inflammatory and Immunomodulatory Mechanism of Tanshinone IIA for Atherosclerosis. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Kamata, K.; Noguchi, M.; Nagai, M. Hypotensive effects of lithospermic acid B isolated from the extract of Salviae miltiorrhizae Radix in the rat. Pharmacol. Vasc. 1994, 25, 69–73. [Google Scholar] [CrossRef]
- Tzen, J.T.C.; Jinn, T.R.; Chen, Y.C.; Li, F.Y.; Cheng, F.C.; Shi, L.S.; She, H.K.; Chen, B.C.; Hsieh, V.; Tu, M.L. Magnesium lithospermate B possesses inhibitory activity on Na+, K+-ATPase and neuroprotective effects against ischemic stroke 1. Acta Pharmacol. Sin. 2007, 28, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Wang, Y.-P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Lo, Y.-H.; Chen, Y.-T.; Lai, N.-W.; Lin, N.-H.; Chung, T.-Y.; Chen, W.-Y.; Tzen, J.T. Magnesium lithospermate B improves metabolic changes in high-fat diet-fed rats with metabolic syndrome. J. Funct. Foods 2015, 14, 163–173. [Google Scholar] [CrossRef]
- Liu, A.-H.; Li, L.; Xu, M.; Lin, Y.-H.; Guo, H.-Z.; Guo, D.-A. Simultaneous quantification of six major phenolic acids in the roots of Salvia miltiorrhiza and four related traditional Chinese medicinal preparations by HPLC–DAD method. J. Pharm. Biomed. Anal. 2006, 41, 48–56. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Shen, J.; Cheng, Y.-Y.; Qu, H.-B. Simultaneous detection of seven phenolic acids in Danshen injection using HPLC with ultraviolet detector. J. Zhejiang Univ. B 2008, 9, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, S.; Putalun, W.; Tsuchihashi, R.; Morimoto, S.; Kinjo, J.; Tanaka, H. Development of an enzyme-linked immunosorbent assay (ELISA) using highly-specific monoclonal antibodies against plumbagin. Anal. Chim. Acta 2008, 607, 100–105. [Google Scholar] [CrossRef]
- Paudel, M.K.; Putalun, W.; Sritularak, B.; Morinaga, O.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Development of a combined technique using a rapid one-step immunochromatographic assay and indirect competitive ELISA for the rapid detection of baicalin. Anal. Chim. Acta 2011, 701, 189–193. [Google Scholar] [CrossRef]
- Paudel, M.K.; Takei, A.; Sakoda, J.; Juengwatanatrakul, T.; Sasaki-Tabata, K.; Putalun, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Preparation of a Single-Chain Variable Fragment and a Recombinant Antigen-Binding Fragment against the Anti-Malarial Drugs, Artemisinin and Artesunate, and Their Application in an ELISA. Anal. Chem. 2012, 84, 2002–2008. [Google Scholar] [CrossRef]
- Singh, K.V.; Kaur, J.; Varshney, G.C.; Raje, M.; Suri, C.R. Synthesis and Characterization of Hapten−Protein Conjugates for Antibody Production against Small Molecules. Bioconjugate Chem. 2004, 15, 168–173. [Google Scholar] [CrossRef]
- Song, J.; Wang, R.-M.; Wang, Y.-Q.; Tang, Y.-R.; Deng, A.-P. Hapten Design, Modification and Preparation of Artificial Antigens. Chin. J. Anal. Chem. 2010, 38, 1211–1218. [Google Scholar] [CrossRef]
- Erlanger, B.F. [4] The preparation of antigenic Hapten-Carrier conjugates: A survey. Methods Enzym. 1980, 70, 85–104. [Google Scholar]
- Parker, D.C. T cell-dependent B cell activation. Annu. Rev. Immunol. 1993, 11, 331–360. [Google Scholar] [CrossRef]
- Hanly, W.C.; Artwohl, J.E.; Bennett, B.T. Review of Polyclonal Antibody Production Procedures in Mammals and Poultry. ILAR J. 1995, 37, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.-E.; Lin, Y.-C.; Chung, T.-Y.; Liu, M.-C.; Chen, G.-H.; Wu, C.-C.; Tzen, J.T. In vitro assay to estimate tea astringency via observing flotation of artificial oil bodies sheltered by caleosin fused with histatin 3. J. Food Drug Anal. 2017, 25, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-T.; Tsai, T.-R.; Lee, C.-Y.; Wei, Y.-S.; Chen, Y.-J.; Chen, C.-R.; Tzen, J.T.C. Elevating Bioavailability of Curcumin via Encapsulation with a Novel Formulation of Artificial Oil Bodies. J. Agric. Food Chem. 2013, 61, 9666–9671. [Google Scholar] [CrossRef]
- Tzen, J.T.C. Integral Proteins in Plant Oil Bodies. ISRN Bot. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-H.; Chyan, C.-L.; Li, F.-Y.; Chen, Y.-J.; Tzen, J.T.C. Engineering lysine-rich caleosins as carrier proteins to render biotin as a hapten on artificial oil bodies for antibody production. Biotechnol. Prog. 2011, 27, 1760–1767. [Google Scholar] [CrossRef]
- Guo, T.; Feng, W.-H.; Liu, X.-Q.; Gao, H.-M.; Wang, Z.-M.; Gao, L.-L. Fourier transform mid-infrared spectroscopy (FT-MIR) combined with chemometrics for quantitative analysis of dextrin in Danshen (Salvia miltiorrhiza) granule. J. Pharm. Biomed. Anal. 2016, 123, 16–23. [Google Scholar] [CrossRef]
- Chung, T.-Y.; Lin, N.-H.; Li, Y.-C.; Chen, T.-Y.; Kuo, P.-C.; Chen, W.-Y.; Tzen, J.T. Detection of lithospermate B in rat plasma at the nanogram level by LC/MS in multi reaction monitoring mode. J. Food Drug Anal. 2018, 26, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chow, M.; Zuo, Z. Improved quality control method for Danshen products—Consideration of both hydrophilic and lipophilic active components. J. Pharm. Biomed. Anal. 2006, 41, 744–750. [Google Scholar] [CrossRef]
- Tian, X.H.; Wu, J.H. Tanshinone derivatives: A patent review (January 2006–September 2012). Expert. Opin. Ther. Pat. 2013, 23, 19–29. [Google Scholar] [CrossRef]
- Lin, N.H.; Chung, T.Y.; Li, F.Y.; Chen, H.A.; Tzen, J.T.C. Enhancing the potency of lithospermate B for inhibiting Na+/K+-ATPase activity by forming transition metal ion complexes. Acta Pharmacol. Sin. 2013, 34, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-H.; Chyan, C.-L.; Li, F.-Y.; Tzen, J.T.C. Stability of Artificial Oil Bodies Constituted with Recombinant Caleosins. J. Agric. Food Chem. 2009, 57, 2308–2313. [Google Scholar] [CrossRef]
- Polson, A.; Coetzer, T.; Kruger, J.; Von Maltzahn, E.; Van Der Merwe, K.J. Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunol. Investig. 1985, 14, 323–327. [Google Scholar] [CrossRef]
- Hatta, H.; Kim, M.; Yamamoto, T. A novel isolation method for hen egg yolk antibody, “IgY”. Agric. Boil. Chem. 1990, 54, 2531–2535. [Google Scholar]
Sample Availability: Sample of Danshen is available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-E.; Chen, C.-H.; Lin, N.-H.; Tzen, J.T.C. Development of Indirect Competitive ELISA for Lithospermic Acid B of Salvia miltiorrhiza with Its Specific Antibodies Generated via Artificial Oil Bodies. Molecules 2019, 24, 1952. https://doi.org/10.3390/molecules24101952
Shih Y-E, Chen C-H, Lin N-H, Tzen JTC. Development of Indirect Competitive ELISA for Lithospermic Acid B of Salvia miltiorrhiza with Its Specific Antibodies Generated via Artificial Oil Bodies. Molecules. 2019; 24(10):1952. https://doi.org/10.3390/molecules24101952
Chicago/Turabian StyleShih, Yu-En, Chao-Hsiang Chen, Nan-Hei Lin, and Jason T.C. Tzen. 2019. "Development of Indirect Competitive ELISA for Lithospermic Acid B of Salvia miltiorrhiza with Its Specific Antibodies Generated via Artificial Oil Bodies" Molecules 24, no. 10: 1952. https://doi.org/10.3390/molecules24101952
APA StyleShih, Y. -E., Chen, C. -H., Lin, N. -H., & Tzen, J. T. C. (2019). Development of Indirect Competitive ELISA for Lithospermic Acid B of Salvia miltiorrhiza with Its Specific Antibodies Generated via Artificial Oil Bodies. Molecules, 24(10), 1952. https://doi.org/10.3390/molecules24101952