Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties
Abstract
:1. Introduction
2. Results
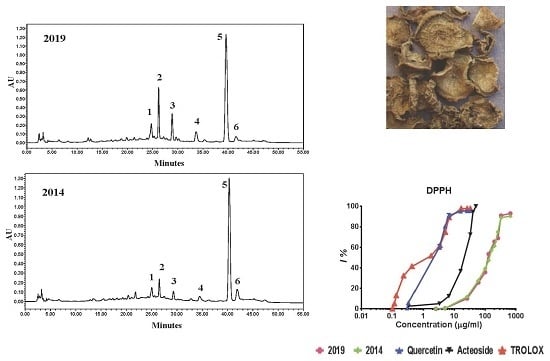
2.1. Chemical Analysis
2.2. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Solvents and Reagents
4.2. Samples
4.3. Thin Layer Chromatography (TLC)
4.4. Harpagoside Isolation
4.5. HPLC-DAD-ELSD
4.6. Validation
4.7. LC-ESI-MS/MS
4.8. Antioxidant Activity
4.9. DPPH
4.10. FRAP
4.11. Trolox/ABTS
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, K.M.; Cole, D. The commercial harvest of devil’s claw (Harpagophytum spp.) in southern Africa: The devil’s in the details. J. Ethnopharmacol. 2005, 100, 225–236. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, B.-E. A broad review of commercially important southern African medicinal plants. J. Ethnopharmacol. 2008, 119, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Wegener, T.; Lüpke, N.-P. Treatment of patients with artrosis of hip and knee with an aqueous extract of devil’s claw (Harpagophytum procumbens D.C.). Phytother. Res. 2003, 17, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- European Union Herbal Monograph on Harpagophytum Procumbens DC. and/or Harpagophytum zeyheri Decne., Radix; EMA/HMPC/627057/2015; European Medicines Agency: Amsterdam, The Netherlands, 2016; pp. 2–8.
- Devil’s Claw Root-Harpagophytum Procumbens DC. and/or Harpagophytum zeyheri Decne., radix; EMA/571858/2016; European Medicines Agency: Amsterdam, The Netherlands, 2016; pp. 1–2.
- Moritz, S. Alchemy and contemporary spagyric medicine: A historical and socio-scientific approach. Interdiscip. Sci. Rev. 2016, 41, 13–27. [Google Scholar] [CrossRef]
- Li, S.; Han, Q.; Qiao, C.; Song, J.; Cheng, C.L.; Xu, H. Chemical markers for the quality control of herbal medicines: An overview. Chinese Med. 2008, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Dai, J.; Wang, W.; Cao, H.; Wu, J.; Gou, X. Quality control method for herbal medicine–Chemical fingerprinting analysis. In Quality Control of Herbal Medicines and Related Areas; Shoyama, Y., Ed.; InTech: Rijeka, Croatia, 2011; pp. 171–194. [Google Scholar] [CrossRef]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprinting and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef]
- Xie, P.; Chen, S.; Liang, Y.; Wang, X.; Tian, R.; Upton, R. Chromatographic fingerprint analysis—A rational approach for quality assessment of traditional Chinese herbal medicine. J. Chromatog. A 2006, 1112, 171–180. [Google Scholar] [CrossRef]
- Reflection Paper on Markers Used for Quantitative and Qualitative Analysis of Herbal Medicinal Products and Traditional Herbal Medicinal Products; EMEA/HMPC/253629/2007; European Medicines Agency: Amsterdam, The Netherlands, 2008; pp. 1–6.
- Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products. Final; European Medicines Agency: Amsterdam, The Netherlands, 2011; pp. 1–13.
- Boje, K.; Lechtenberg, M.; Nahrstedt, A. New and known iridoid- and phenylethanoid glycosides from Harpagophytum procumbens and their in vitro inhibition of human leukocyte elastase. Planta Med. 2003, 69, 820–825. [Google Scholar]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.M.; Gericke, N. Devil’s claw–A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef]
- Viljioen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Ahmed, B.; Al-Rehaily, A.J.; Al-Howiriny, T.A.; El-Sayed, K.A.; Ahmad, M.S. Scropoloside D2 and harpagoside–B: Two new iridoid glycosides from Scrophularia deserti and their antidiabetic and anti-inflammatory activity. Biol. Pharm. Bull. 2003, 26, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.F.; Peroza, L.R.; Boligon, A.A.; Althayde, M.L.; Alves, S.H.; Faschinetto, R.; Wagner, C. Harpagophytum procumbens prevents oxidative stress and loss of cell viability in vitro. Neurochem. Res. 2013, 38, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia (Ph. Eur.) 9th Edition. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition (accessed on 26 February 2019).
- Li, L.; Tsao, R.; Liu, Z.; Liu, S.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and purification of acteoside and isoacteoside from Plantago psyllium L. by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.-T.; Fontaine, J.; Malonne, H.; Clayes, M.; Luhmer, M.; Duez, P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry 2005, 66, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.; Staerk, D.; Hansen, S.H.; Smith, P.J.; Jaroszewski, J.W. Identification of major and minor constituents of Harpagophytum procumbens (Devil’s claw) using HPLC-SPE-NMR and HPLC-ESIMS/APCIMS. J. Nat. Prod. 2006, 69, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yuan, Q.; Liu, E.-H.; Qi, L.-W.; Bi, Z.-M.; Li, P. Fragmentation study of iridoid glycosides and phenylpropanoid glycosides in Radix scrophulariae by rapid-resolution liquid chromatography with diode-array detection and electrospray ionization time-of-flight mass spectrometry. Biomed. Chromatogr. 2010, 24, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Fani, E.; Vincieri, F.F.; Bilia, A.R. Analysis and stability of the constituents of Curcuma longa and Harpagophytum procumbens tinctures by HPLC-DAD and HPLC-ESI.MS. J. Pharm. Biomed. Anal. 2011, 55, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, J.-J.; Cheng, Z.-H.; Zhou, J.-H.; Yu, B.-Y.; Qiu, S.X. Iridoid glycosides from Harpagophytum procumbens DC. (devil’s claw). Phytochemistry 2006, 67, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Ivanovska, N.; Alipieva, K.; Dimitrova, P.; Veerporte, R. Harpagoside: From Kalahari desert to pharmacy shelf. Phytochemistry 2013, 92, 8–15. [Google Scholar] [CrossRef]
- Kikuchi, T.; Matsuda, S.; Kubo, Y.; Namba, T. New iridoids glucoisdes from Harpagophytum procumbens DC. Chem. Pharm. Bull. 1983, 31, 2296–2301. [Google Scholar] [CrossRef]
- Schopohl, P.; Grüneberg, P.; Melzig, M.F. The influence of harpagoside and harpagide on TNFα-secretion and cell adhesion molecule mRNA-expression in IFNγ/LPS-stimulated THP-1 cells. Fitoterapia 2016, 110, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines, 3rd ed.; Pharmaceutical Press: London, UK, 2007; pp. 207–214. [Google Scholar]
- Zhang, L.; Fenbg, L.-F.; Jia, Q.; Xu, J.; Wang, R.; Wang, Z.; Wu, Y.; Li, Y. Effects of ß-glucisidase hydrolyzed products of harpagide and harpagoside on cyclooxygenase-2 (COX-2) in vitro. Biorg. Med. Chem. 2011, 19, 4882–4886. [Google Scholar] [CrossRef] [PubMed]
- Arthur, H.; Joubert, E.; De Beer, D.; Malherbe, C.J.; Witthuhn, R.C. Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurization of plant material. Food Chem. 2011, 127, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Jenke, D.R. Chromatographic method validation: A review of current practices and procedures. II. Guidelines for primary validation parameters. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 737–757. [Google Scholar] [CrossRef]
- Vial, J.; Jardy, A. Experimental comparison of the different approaches to estimate LOD and LOQ for an HPLC method. Anal. Chem. 1999, 71, 2672–2677. [Google Scholar] [CrossRef]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compo. Anal. 2006, 19, 669–6775. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Sample Availability: Samples of the tinctures 019, 014 and NST are available from the authors. |




| Number * | Rt (min) | Name | UV (λ max, nm) | ms1 | ms2 |
|---|---|---|---|---|---|
| m/z (%) | |||||
| 1 | 24.70 | Acteoside | 216.6, 246.5 sh, 290.5 sh, 325.7 | 623.0 (100) [M − H]− | 461.0 (100) [(M − H)-162]−; 315.0 (4) [(M − H)-162-146]−; 179.0 (2), [caffeoyl moiety] − |
| 2 | 26.20 | Isoacteoside | 217.7, 248.5 sh, 292.3 sh, 326.9 | 623.0 (100) [ M − H ]− | 461.0 (100) [(M-H)-162]−; 315.0 (4) [(M − H)-162-146]−; 179.0 (2), [caffeoyl moiety]− |
| 3 | 28.87 | 8-O-p-Coumaroyl-harpagide | 224.8, 311.4 | 533.1 (100) [M + Na]+ | 369.2 (100) [(M + Na)-164]+; 351.0 (44) [(M + Na)-164-18]+; 203.0 (6) [harpagide-162]+ |
| 4 | 33.60 | Pagoside | 224.8, 311.4 | 491.0 (100) [ M − H ]− | 311.2 (100) [(M − H)-162-18]−; 163.0 (31) [coumaric acid]− |
| 5 | 39.58 | Harpagoside | 217.7, 280.4 | 1011.5 (5) [2M + Na]+ /517.2 (100) [M + Na]+ | 369.2 (100) [(M + Na)-148]+; 351.0 (44) [(M + Na)-148-18]+; 203.0 (6) [(M + Na)-148-166]+ |
| 6 | 41.64 | 8-Cinnamoylmyoporoside | 217.7, 278.1 | 501.0 (100) [M + Na]+ | 353.1 (100) [(M + Na)-148]+; 335.2 (69) [(M + Na)-148-18]+; 339.0 (41) [(M + Na)-162]+; 203.0 (6) harpagide-162]+ |
| Compound | 014 | 019 | NST | |||
|---|---|---|---|---|---|---|
| μg/mg ext | % | μg/mg ext | % | μg/mg ext | % | |
| 1 | 1.49 ± 0.02 | 7.59 | 1.99 ± 0.23 | 7.90 | 1.35 ± 0.15 | 7.39 |
| 2 | 1.87 ± 0.14 | 9.52 | 3.94 ± 0.006 | 15.65 | 1.55 ± 0.04 | 8.48 |
| 3 | 0.68 ± 0.006 | 3.46 | 2.21 ± 0.0003 | 8.78 | 0.95 ± 0.04 | 5.2 |
| 4 | 0.53 ± 0.009 | 2.70 | 1.37 ± 0.01 | 5.44 | 0.72 ± 0.02 | 3.94 |
| 5 | 13.68 ± 0.19 | 69.68 | 14.97 ± 0.09 | 59.47 | 13.25 ± 0.07 | 72.52 |
| 6 | 1.38 ± 0.009 | 7.03 | 0.69 ± 0.008 | 2.74 | 0.45 ± 0.005 | 2.46 |
| Sample | IC50 (mEq Trolox) ± SD | ||
|---|---|---|---|
| DPPH | ABTS | FRAP | |
| 019 | 92.53 ± 0.31 | 22.89 ± 0.19 | 14.11 ± 0.08 |
| 014 | 93.33 ± 0.25 | 19.49 ± 0.13 | 6.69 ± 0.07 |
| NST | 83.02 ± 0.28 | 17.78 ± 0.12 | 11.32 ± 0.08 |
| Acteoside | 13.83 ± 0.09 | 0.96 ± 0.05 | 0.44 ± 0.04 |
| Quercetin | 1.38 ± 0.01 | 0.60 ± 0.07 | 0.38 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avato, P.; Argentieri, M.P. Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties. Molecules 2019, 24, 2251. https://doi.org/10.3390/molecules24122251
Avato P, Argentieri MP. Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties. Molecules. 2019; 24(12):2251. https://doi.org/10.3390/molecules24122251
Chicago/Turabian StyleAvato, Pinarosa, and Maria Pia Argentieri. 2019. "Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties" Molecules 24, no. 12: 2251. https://doi.org/10.3390/molecules24122251
APA StyleAvato, P., & Argentieri, M. P. (2019). Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties. Molecules, 24(12), 2251. https://doi.org/10.3390/molecules24122251