Exploration of Li-Organic Batteries Using Hexaphyrin as an Active Cathode Material
Abstract
:1. Introduction
2. Results and Discussion
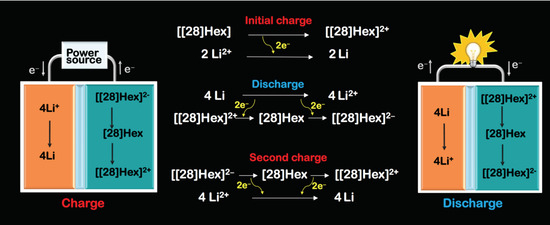
2.1. Preparation of Li-[28]Hexaphyrin Batteries and Investigation of the Battery Behaviors
2.2. Absroption Spectroscopy with Electrochemical Redox Reaction
2.3. Further Battery Performances
3. Materials and Methods
3.1. Preparation of Li-[28]hex Batteries
3.2. Optimization of the Current Window for Best Charge/Discharge Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scherson, D.A.; Palencsár, A. Batteries and electrochemical capacitors. Interface 2006, 15, 17–22. [Google Scholar]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-M. Building a better battery. Science 2010, 330, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Noorden, R.V. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Derhardt, M.R.; Calvin, C.J.; Chen, X.; Aspuru-Gusik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic-inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tao, Z.; Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Liu, X.; Zhang, T.; Zhu, Y.; Yu, H.; Otani, M.; Zhou, H. A quinone-based oligomeric lithium salt for superior Li-organic batteries. Energy Environ. Sci. 2014, 7, 4077–4086. [Google Scholar]
- Yoon, Z.S.; Osuka, A.; Kim, D. Möbius aromaticity and antiaromaticity in expanded porphyrins. Nat. Chem. 2009, 1, 113–122. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Chemistry of meso-aryl-substituted expanded porphyrins: Aromaticity and molecular twist. Chem. Rev. 2017, 4, 2584–2640. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Furuta, H.; Osuka, A. N-Fused pentaphyrin. Angew. Chem. 2001, 113, 639–641, reprinted in Angew. Chem. Int. Ed. 2001, 40, 619–621. [Google Scholar]
- Ito, T.; Hayashi, Y.; Shimizu, S.; Shin, J.-Y.; Kobayashi, N.; Shinokubo, H. Gram-scale synthesis of nickel(II) norcorrole: The smallest antiaromatic porphyrinoid. Angew. Chem. 2012, 124, 8670–8673, reprinted in Angew. Chem. Int. Ed. 2012, 51, 8542–8545. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Yamada, T.; Yoshikawa, H.; Awaga, K.; Shinokubo, H. An antiaromatic electrode-active material enabling high capacity and stable performance of rechargeable batteries. Angew. Chem. 2014, 126, 3160–3165, reprinted in Angew. Chem. Int. Ed. 2014, 53, 3096–3101. [Google Scholar] [CrossRef]
- Fujii, S.; Margués-González, S.; Shin, J.-Y.; Shinokubo, H.; Masuda, T.; Nishino, T.; Arasu, N.P.; Vázquez, H.; Kiguchi, M. High-conducting molecular circuits based on antiaromaticity. Nat. Comm. 2017, 8, 15984. [Google Scholar] [CrossRef] [PubMed]
- Jasat, A.; Dolphin, D. Expanded porphyrins and their heterologs. Chem. Rev. 1997, 97, 2267–2340. [Google Scholar] [CrossRef]
- Sessler, J.L.; Hemmi, G.; Mody, T.D.; Turai, T.; Burrell, A.; Young, S.W. Texaphyrins: Synthesis and applications. Acc. Chem. Res. 1994, 27, 43–50. [Google Scholar] [CrossRef]
- Newell, L.C. Faraday’s discovery of benzene. J. Chem. Educ. 1926, 3, 1248–1253. [Google Scholar] [CrossRef]
- Heilbronner, E. Hückel molecular orbitals of Möbius-type conformations. Tetrahedron lett. 1923, 29, 1923–1928. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Stanger, A. Magnetic criteria of aromaticity. Chem Soc. Rev. 2015, 44, 6597–6615. [Google Scholar] [CrossRef]
- Saito, S.; Osuka, A. Expanded porphyrins: Intriguing structures, electronic properties, and reactivities. Angew. Chem. Int. Ed. 2011, 50, 4342–4373. [Google Scholar] [CrossRef]
- Saito, S.; Osuka, A. Expanded porphyrins and aromaticity. Chem. Commun. 2011, 47, 4330–4339. [Google Scholar]
- Sessler, J.L.; Weghorn, S.; Lynch, V.; Johnson, M.R. Turcasarin, the largest expanded porphyrin to date. Angew. Chem. 1994, 106, 1572–1575, reprinted in Angew. Chem. Int. Ed. 1994, 33, 1509–1512. [Google Scholar] [CrossRef]
- Stȩpień, M.; Latos-Grażyński, L. Benziporphyrins: Exploring arene chemistry in a macrocyclic environment. Acc. Chem. Res. 2005, 38, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Stȩpień, M.; Latos-Grażyński, L. Tetraphenyl-p-benziporphyrin: A carbaporphyrinoid with two linked carbon atoms in the coordination core. J. Am. Chem. Soc. 2002, 124, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Naoda, K.; Mori, H.; Oh, J.; Park, K.H.; Kim, D.; Osuka, A. 5,20-Di(priding-2-yl)-[28]hexaphyrin(1.1.1.1.1.1): A stable Hückel antiaromatic hexaphyrin stabilized by intramolecular hydrogen bonding and protonation-induced conformational twist to gain Möbius aromaticity. J. Org. Chem. 2015, 80, 11726–11733. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.M.; Oh, J.; Kim, W.; Mori, H.; Osuka, A.; Kim, D. Switching between aromatic and antiaromatic 1,3-phenylene-strapped [26]- and [28]hexaphyrin upon passage to the singlet excited state. J. Am. Chem. Soc. 2015, 137, 11856–11859. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.M.; Yoon, M.-C.; Lim, J.M.; Rath, H.; Naoda, K.; Osuka, A.; Kim, D. Reversal of Hückel (anti)aromaticity in the lowest triplet states of hexaphyrins and spectroscopic evidence for Baird’s rule. Nat. Chem. 2015, 7, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Soya, T.; Kim, W.; Kim, D.; Osuka, A. A Möbius aromatic [28]hexaphyrin bearing a diethylamine group: A rigid but smooth conjugation circuit. Angew. Chem. Int. Ed. 2015, 54, 5456–5459. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Furuta, H.; Yoza, K.; Igarashi, S.; Osuka, A. meso-Aryl-substituted expanded porphyrins. J. Am. Chem. Soc. 2001, 123, 7190–7191. [Google Scholar] [CrossRef]
- Neves, M.G.P.M.S.; Martins, R.M.; Tomé, A.C.; Silvestre, A.J.D.; Silva, A.M.S.; Félix, V.; Drew, M.G.B.; Cavaleiro, J.A.S. meso-Substituted expanded porphyrins: New and stable hexaphyrins. Chem. Commun. 1999, 385–386. [Google Scholar] [CrossRef]
- Wang, C.; Sawicki, M.; Emani, S.; Liu, C.; Shaw, L.L. Na3MnCO3PO4—A high capacity multi-electron transfer redox cathode material for sodium ion batteries. Electrochimica Acta 2015, 161, 322–328. [Google Scholar] [CrossRef]
- Wang, C.; Sawicki, M.; Kaduk, J.A.; Shaw, L.L. Roles of processing, structural defects and ionic conductivity in the electrochemical performance of Na3MnCO3PO4 cathode material. J. Electrochem. Soc. 2015, 162, A1601–A1609. [Google Scholar] [CrossRef]
- Taniguchi, R.; Shimizu, S.; Suzuki, M.; Shin, J.-Y.; Furuta, H.; Osuka, A. Ring size selective synthesis of meso-aryl expanded porphyrins. Tetrahedron Lett. 2003, 44, 2505–2507. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound [28]hexaphyrin is available from the authors. |









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-Y.; Zhang, Z.; Awaga, K.; Shinokubo, H. Exploration of Li-Organic Batteries Using Hexaphyrin as an Active Cathode Material. Molecules 2019, 24, 2433. https://doi.org/10.3390/molecules24132433
Shin J-Y, Zhang Z, Awaga K, Shinokubo H. Exploration of Li-Organic Batteries Using Hexaphyrin as an Active Cathode Material. Molecules. 2019; 24(13):2433. https://doi.org/10.3390/molecules24132433
Chicago/Turabian StyleShin, Ji-Young, Zhongyue Zhang, Kunio Awaga, and Hiroshi Shinokubo. 2019. "Exploration of Li-Organic Batteries Using Hexaphyrin as an Active Cathode Material" Molecules 24, no. 13: 2433. https://doi.org/10.3390/molecules24132433
APA StyleShin, J. -Y., Zhang, Z., Awaga, K., & Shinokubo, H. (2019). Exploration of Li-Organic Batteries Using Hexaphyrin as an Active Cathode Material. Molecules, 24(13), 2433. https://doi.org/10.3390/molecules24132433