Sonochemically-Promoted Preparation of Silica-Anchored Cyclodextrin Derivatives for Efficient Copper Catalysis
Abstract
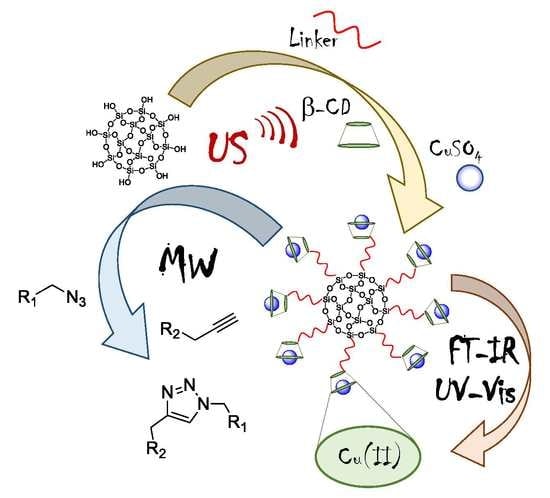
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Copper Supported on Si-NH-CD and Si-DETA-CD
2.2. Characterization of Copper Supported on Si-NH-CD and Si-DETA-CD
2.3. Test of the Catalytic Activity of Copper Supported on Si-NH-CD and Si-DETA-CD
2.4. Synthesis, Characterization, and Catalytic Activity of Copper Supported on Si-diAm-CD
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Preparation of Chlorinate Silica
Si-Cl Titration
3.2.2. Preparation of Si-DETA
3.2.3. Preparation of Si-DETA-CD
3.2.4. Preparation of Si-NH-CD
3.2.5. Preparation of Si-DiAm
3.2.6. Preparation of Si-DiAm-CD
3.2.7. Cyclodextrin-Supported Silica and Copper Complexation
3.3. Click Chemistry Reaction
3.4. Physico-Chemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomez-Romero, P. Hybrid organic-inorganic materials—In search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Kim, H.J.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 487–492. [Google Scholar] [CrossRef]
- Parola, S.; Julian-Lopez, B.; Carlos, L.D.; Sanchez, C. Optical properties of hybrid organic-inorganic materials and their applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Taleghani, S.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. Tuning the topology of hybrid inorganic-organic materials based on the study of flexible ligands and negative charge of polyoxometalates: A crystal engineering perspective. Coord. Chem. Rev. 2016, 309, 84–106. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Organic-inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Wan, C.L.; Tian, R.M.; Kondou, M.; Yang, R.G.; Zong, P.G.; Koumoto, K. Ultrahigh thermoelectric power factor in flexible hybrid inorganic-organic superlattice. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Zhang, C.; Liang, Y.; Zhang, Q.; Fu, P.; Liu, M.; Zhao, Q.; Cui, Z.; Pang, X. Facile fabrication of size-tunable core/shell ferroelectric/polymeric nanoparticles with tailorable dielectric properties via organocatalyzed atom transfer radical polymerization driven by visible light. Sci. Rep. 2019, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Soler-Illia, G.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Lashgari, N.; Badiei, A. Sulfonic acid-functionalized mesoporous silica (SBA-Pr-SO3H) as solid acid catalyst in organic reactions. J. Mol. Catalys. Chem. 2015, 397, 166–191. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Badiei, A.; Mousavi, S.; Lashgari, N.; Shahbazi, A. Application of amino-functionalized SBA-15 type mesoporous silica in one-pot synthesis of spirooxindoles. Chin. J. Catalys. 2012, 33, 1832–1839. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Chatterjee, S.; Bhaumik, A. Bifunctionalized mesoporous SBA-15: A new heterogeneous catalyst for the facile synthesis of 5-hydroxymethylfurfural. Acs Sustain. Chem. Eng. 2017, 5, 2763–2773. [Google Scholar] [CrossRef]
- Bied, C.; Gauthier, D.; Moreau, J.J.E.; Man, M.W.C. Preparation and characterization of new templated hybrid materials containing a chiral diamine ligand. J. Sol-Gel Sci. Technol. 2001, 20, 313–320. [Google Scholar] [CrossRef]
- Du, J.Z.; Chen, Y.M. Organic-inorganic hybrid nanoparticles with a complex hollow structure. Angew. Chem. Int. Ed. 2004, 43, 5084–5087. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.; Favette, S.; Chamoreau, L.M.; Vaissermann, J.; Ruhlmann, L.; Hasenknopf, B. Hybrid organic-inorganic porphyrin-polyoxometalate complexes. Eur. J. Inorg. Chem. 2008, 3433–3441. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, P.P.; Shen, Y.R.; Zhang, W.J.; Bian, G. Covalent anchoring of Mo(VI) Schiff base complex into SBA-15 as a novel heterogeneous catalyst for enhanced alkene epoxidation. J. Porous Mater. 2016, 23, 431–440. [Google Scholar] [CrossRef]
- Zare, M.; Moradi-Shoeili, Z.; Ashouri, F.; Bagherzadeh, M. Heterogeneous SBA-15-supported oxoperoxomolybdenum(VI) complex for enhanced olefin epoxidation. Catal. Commun. 2017, 88, 9–12. [Google Scholar] [CrossRef]
- Bhar, S.; Ananthakrishnan, R. Ru(II)-metal complex immobilized mesoporous SBA-15 hybrid for visible light induced photooxidation of chlorophenolic compounds in aqueous medium. Photochem. Photobiol. Sci. 2017, 16, 1290–1300. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in metal recycling. Science 2012, 337, 690. [Google Scholar] [CrossRef]
- Song, B.Q.; Wang, X.L.; Sun, C.Y.; Zhang, Y.T.; Wu, X.S.; Yang, L.; Shao, K.Z.; Zhao, L.; Su, Z.M. An organic-inorganic hybrid photocatalyst based on sandwich-type tetra-Co-substituted phosphotungstates with high visible light photocatalytic activity. Dalton Trans. 2015, 44, 13818–13822. [Google Scholar] [CrossRef]
- Wei, X.L.; Lu, X.H.; Ma, X.T.; Peng, C.; Jiang, H.Z.; Zhou, D.; Xia, Q.H. Synthesis and catalytic activity of organic-inorganic hybrid catalysts coordinated with cobalt(II) ions for aerobic epoxidation of styrene. Catal. Commun. 2015, 61, 48–52. [Google Scholar] [CrossRef]
- Barbe, J.M.; Canard, G.; Brandes, S.; Guilard, R. Organic-inorganic hybrid sol-gel materials incorporating functionalized cobalt(III) corroles for the selective detection of CO. Angew. Chem. Int. Ed. 2005, 44, 3103–3106. [Google Scholar] [CrossRef] [PubMed]
- Al-Rehili, S.; Fhayli, K.; Hammami, M.A.; Moosa, B.; Patil, S.; Zhang, D.L.; Alharbi, O.; Hedhili, M.N.; Mohwald, H.; Khashab, N.M. Anisotropic self-assembly of organic-inorganic hybrid microtoroids. J. Am. Chem. Soc. 2017, 139, 10232–10238. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- Hojoh, K.; Ohmiya, H.; Sawamura, M. Synthesis of alpha-quaternary formimides and aldehydes through umpolung asymmetric copper catalysis with isocyanides. J. Am. Chem. Soc. 2017, 139, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lu, L.Q.; Yao, S.; Chen, J.R.; Shi, D.Q.; Xiao, W.J. Enantioconvergent copper catalysis: In situ generation of the chiral phosphorus ylide and its wittig reactions. J. Am. Chem. Soc. 2017, 139, 12847–12854. [Google Scholar] [CrossRef] [PubMed]
- Hickman, A.J.; Sanford, M.S. High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Meyer, T.J. Copper(II) Catalysis of water oxidation. Angew. Chem. Int. Ed. 2013, 52, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Zhu, N.; Chen, P.H.; Lin, Z.Y.; Liu, G.S. Enantioselective decarboxylative cyanation employing cooperative photoredox catalysis and copper catalysis. J. Am. Chem. Soc. 2017, 139, 15632–15635. [Google Scholar] [CrossRef]
- Saunthwal, R.K.; Patel, M.; Kumar, S.; Verma, A.K. Cu(II)-catalyzed tandem synthesis of 2-imino 1,3 benzothiazines from 2-aminoaryl acrylates via thioamidation and concomitant chemoselective thia-Michael addition. Tetrahedron Lett. 2015, 56, 677–681. [Google Scholar] [CrossRef]
- Zhang, G.F.; Han, X.W.; Luan, Y.X.; Wang, Y.; Wen, X.; Xu, L.; Ding, C.R.; Gao, J.R. Copper-catalyzed aerobic alcohol oxidation under air in neat water by using a water-soluble ligand. Rsc Adv. 2013, 3, 19255–19258. [Google Scholar] [CrossRef]
- Maity, R.; Naskar, S.; Das, I. Copper(II)-catalyzed reactions of alpha-keto thioesters with azides via C–C and C–S bond cleavages: Synthesis of N-acylureas and amides. J. Org. Chem. 2018, 83, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Ragauskas, A.J. Cu(II)-catalyzed selective aerobic oxidation of alcohols under mild conditions. J. Org. Chem. 2006, 71, 7087–7090. [Google Scholar] [CrossRef] [PubMed]
- Kitanosono, T.; Xu, P.Y.; Kobayashi, S. Heterogeneous versus homogeneous copper(II) catalysis in enantioselective conjugate-addition reactions of boron in water. Chem. Asian J. 2014, 9, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Liu, H.J.; Lo, H.K.; Coperet, C. Silica-supported cu nanoparticle catalysts for alkyne semihydrogenation: Effect of ligands on rates and selectivity. J. Am. Chem. Soc. 2016, 138, 16502–16507. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.Q.; Chen, B.H.; Jing, Z.Q.; Zhao, P.Q. Copper supported on H+-modified manganese oxide octahedral molecular sieves (Cu/H-OMS-2) as a heterogeneous biomimetic catalyst for the synthesis of imidazo 1,2-a -N-heterocycles. Catal. Sci. Technol. 2016, 6, 890–896. [Google Scholar] [CrossRef]
- Zou, Y.D.; Wang, X.X.; Ai, Y.J.; Liu, Y.H.; Ji, Y.F.; Wang, H.Q.; Hayat, T.; Alsaedi, A.; Hu, W.P.; Wang, X.K. beta-Cyclodextrin modified graphitic carbon nitride for the removal of pollutants from aqueous solution: Experimental and theoretical calculation study. J. Mater. Chem. A 2016, 4, 14170–14179. [Google Scholar] [CrossRef]
- Kaboudin, B.; Abedi, Y.; Yokomatsu, T. Cu-II-beta-cyclodextrin complex as a nanocatalyst for the homo- and cross-coupling of arylboronic acids under ligand- and base-free conditions in air: Chemoselective cross-coupling of arylboronic acids in water. Eur. J. Org. Chem. 2011, 6656–6662. [Google Scholar] [CrossRef]
- Gupta, D.; Mishra, A.; Kundu, S. Cu (II)-beta-CD as water-loving catalyst for one-pot synthesis of triazoles and biofuels intermediate at room temperature without any other additive. Chemistryselect 2017, 2, 2997–3008. [Google Scholar] [CrossRef]
- Kaboudin, B.; Abedi, Y.; Yokomatsu, T. One-pot synthesis of 1,2,3-triazoles from boronic acids in water using Cu(II)-beta-cyclodextrin complex as a nanocatalyst. Org. Biomol. Chem. 2012, 10, 4543–4548. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Liu, C.Q.; Chen, C.C.; Yao, Y.G. One-pot synthesis of cyclodextrin-doped Cu-SiO2 catalysts for efficient hydrogenation of dimethyl oxalate to ethylene glycol. Chemcatchem 2017, 9, 4587–4597. [Google Scholar] [CrossRef]
- Duan, Z.B.; Ding, X.C.; Wang, Y.; Zhu, L.J.; Xia, D.H. A new strategy for fuel desulfurization by molecular inclusion with copper(II)-beta-cyclodextrin@SiO2@Fe3O4 for removing thiophenic sulfides. Energy Fuels 2018, 32, 11421–11431. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Ma'mani, L.; Mahdavi, H.; Shafiee, A. Copper supported β-cyclodextrin functionalized PEGylated mesoporous silica nanoparticle -graphene oxide hybrid: An efficient and recyclable nano-catalyst for straightforward synthesis of 2-arylbenzimidazoles and 1,2,3-triazoles. Microporous Mesoporous Mater. 2018, 262, 207–216. [Google Scholar] [CrossRef]
- Saurabh, P.; Balbir, K.; Anupama, P.; Harish, K. Applications of ultrasound in organic synthesis—A green approach. Curr. Org. Chem. 2013, 17, 1790–1828. [Google Scholar]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Borretto, E.; Medlock, J.; Bonrath, W.; Cravotto, G. Effects of ultrasound and microwaves on selective reduction: Catalyst preparation and reactions. ChemCatChem 2014, 6, 2762–2783. [Google Scholar] [CrossRef]
- Luche, J.-L. Effect of ultrasound on heterogeneous systems. Ultrason. Sonochem. 1994, 1, S111–S118. [Google Scholar] [CrossRef]
- Wu, Z.; Tagliapietra, S.; Giraudo, A.; Martina, K.; Cravotto, G. Harnessing cavitational effects for green process intensification. Ultrason. Sonochem. 2019, 52, 530–546. [Google Scholar] [CrossRef]
- Varma, R.S. “Greener” chemical syntheses using mechanochemical mixing or microwave and ultrasound irradiation. Green Chem. Lett. Rev. 2007, 1, 37–45. [Google Scholar] [CrossRef]
- Shaabani, A.; Sepahvand, H.; Hooshmand, S.E.; Borjian Boroujeni, M. Design, preparation and characterization of Cu/GA/Fe3O4@SiO2 nanoparticles as a catalyst for the synthesis of benzodiazepines and imidazoles. Appl. Organomet. Chem. 2016, 30, 414–421. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Wu, C.-C.; Wu, S.-M.; Su, C.-C. Synthesis and properties of carbon nanotube-grafted silica nanoarchitecture-reinforced poly(Lactic Acid). Materials (Basel) 2017, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Gorbachuk, V.V.; Ziatdinova, R.V.; Evtugyn, V.G.; Stoikov, I.I. Stabilization of silica nanoparticles dispersions by surface modification with silicon derivative of thiacalix[4]arene. J. Nanopart. Res. 2015, 17, 117. [Google Scholar] [CrossRef]
- Unterlass, M.M. Green synthesis of inorganic–organic hybrid materials: State of the art and future perspectives. Eur. J. Inorg. Chem. 2016, 2016, 1135–1156. [Google Scholar] [CrossRef]
- Martina, K.; Baricco, F.; Berlier, G.; Caporaso, M.; Cravotto, G. Efficient green protocols for preparation of highly functionalized β-cyclodextrin-grafted silica. Acs Sustain. Chem. Eng. 2014, 2, 2595–2603. [Google Scholar] [CrossRef]
- Martina, K.; Baricco, F.; Caporaso, M.; Berlier, G.; Cravotto, G. Cyclodextrin-grafted silica-supported Pd nanoparticles: An efficient and versatile catalyst for ligand-free C−C coupling and hydrogenation. ChemCatChem 2016, 8, 1176–1184. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Panahi, F. Immobilized palladium nanoparticles on a silica-starch substrate (PNP-SSS): As an efficient heterogeneous catalyst for Heck and copper-free Sonogashira reactions in water. Green Chem. 2011, 13, 2408–2415. [Google Scholar] [CrossRef]
- Mohanazadeh, F.; Amini, H. Silica chloride mediated alkylation of electron-rich aromatics by benzyl or tert-butyl chloride. Bull. Korean Chem. Soc. 2010, 31, 3038–3040. [Google Scholar] [CrossRef]
- Bagabas, A.A.; Frasconi, M.; Iehl, J.; Hauser, B.; Farha, O.K.; Hupp, J.T.; Hartlieb, K.J.; Botros, Y.Y.; Stoddart, J.F. γ-Cyclodextrin cuprate sandwich-type complexes. Inorg. Chem. 2013, 52, 2854–2861. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M.; Zhiani, R.; Moradi, M. KCC-1 Supported Cu(II)-β-cyclodextrin complex as a reusable catalyst for the synthesis of 3-Aryl-2-oxazolidinones from carbon dioxide, epoxide, anilines. ChemistrySelect 2018, 3, 3516–3522. [Google Scholar] [CrossRef]
- Fedorova, A.A.; Morozov, I.V.; Kotovshchikov, Y.N.; Romanovsky, B.V.; Sirotin, S.V.; Knyazeva, E.E.; Lermontov, A.S.; Shaporev, A.S. Preparation and characterization of copper- and iron-containing mesoporous silica using β-cyclodextrin as a structure-directing agent. Mendeleev Commun. 2011, 21, 171–172. [Google Scholar] [CrossRef]
- Liu, J.; He, P.; Wang, L.; Liu, H.; Cao, Y.; Li, H. An efficient and stable Cu/SiO2 catalyst for the syntheses of ethylene glycol and methanol via chemoselective hydrogenation of ethylene carbonate. Chin. J. Catal. 2018, 39, 1283–1293. [Google Scholar] [CrossRef]
- Musso, G.E.; Bottinelli, E.; Celi, L.; Magnacca, G.; Berlier, G. Influence of surface functionalization on the hydrophilic character of mesoporous silica nanoparticles. PCCP 2015, 17, 13882–13894. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How to prepare reproducible, homogeneous, and hydrolytically stable aminosilane-derived layers on silica. Langmuir 2012, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Negri, C.; Signorile, M.; Porcaro, N.G.; Borfecchia, E.; Berlier, G.; Janssens, T.V.W.; Bordiga, S. Dynamic CuII/CuI speciation in Cu-CHA catalysts by in situ Diffuse Reflectance UV–vis-NIR spectroscopy. Appl. Catal. A: Gen. 2019, 578, 1–9. [Google Scholar] [CrossRef]
- Velasco, M.I.; Krapacher, C.R.; de Rossi, R.H.; Rossi, L.I. Structure characterization of the non-crystalline complexes of copper salts with native cyclodextrins. Dalton Trans. 2016, 45, 10696–10707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotta, F.; Martina, K.; Robaldo, B.; Barge, A.; Cravotto, G. Recent advances in the synthesis of cyclodextrin derivatives under microwaves and power ultrasound. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 3–7. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Si-diAm-CD Si-DETA-CD, Si-NH-CD and relative Cu supported catalyst are available from the authors. |











| Entry | Product | Reaction Condition | Time | Loading (w/w %) | Loading (μmol/g) |
|---|---|---|---|---|---|
| 1 | Si-DETA | Si-Cl, DETA, DCM, reflux | 12 h | 14 (a) | 1.37 × 103 (c) |
| 2 | Si-DETA | Si-Cl, DETA, DMF, 60 °C | 12 h | 10 (a) | 0.96 × 103 (c) |
| 3 | Si-DETA | Si-Cl, DETA, toluene, 60 °C | 12 h | 15 (a) | 1.49 × 103 (c) |
| 4 | Si-DETA | Si-Cl, DETA, toluene, US, 40 kHz | 2 h | 9 (a) | 0.87 × 103 (c) |
| 5 | Si-DETA | Si-Cl, DETA, toluene, US, 80 kHz | 2 h | 14 (a) | 1.35 × 103 (c) |
| 6 | Si-DETA | Si-Cl, DETA, toluene, US, 120 kHz | 2 h | 11.5 (a) | 1.12 × 103 (c) |
| 7 | Si-DETA | Si-Cl, DETA, neat, US, 40 kHz | 2 h | 7.1 (a) | 0.69 × 103 (c) |
| 8 | Si-DETA | Si-Cl, DETA, neat, US, 80 kHz | 2 h | 13 (a) | 1.26 × 103 (c) |
| 9 | Si-DETA | Si-Cl, DETA, neat, US, 120 kHz | 2 h | 8.2 (a) | 0.79 × 103 (c) |
| 10 | Si-DETA | Si-Cl, DETA, toluene, US, 40 kHz | 4 h | 10 (a) | 0.97 × 103 (c) |
| 11 | Si-DETA | Si-Cl, DETA, toluene, US, 80 kHz | 4 h | 14.3 (a) | 1.12 × 103 (c) |
| 12 | Si-DETA | Si-Cl, DETA, toluene, US, 120 kHz | 4 h | 10.9 (a) | 0.97 × 103 (c) |
| 13 | Si-DETA | Si-Cl, DETA, neat, US, 40 kHz | 4 h | 8 (a) | 0.77 × 103 (c) |
| 14 | Si-DETA | Si-Cl, DETA, neat, US, 80 kHz | 4 h | 13.5 (a) | 1.26 × 103 (c) |
| 15 | Si-DETA | Si-Cl, DETA, neat, US, 120 kHz | 4 h | 9 (a) | 0.88 × 103 (c) |
| 16 | Si-DETA-CD | Si-DETA, 6-tosyl β-CD, DMF, 70 °C | 24 h | 1.3 (b) | 11 (d) |
| 17 | Si-DETA-CD | Si-DETA, 6-tosyl β-CD, DMF, US, 80 kHz | 4 h | 3.3 (a)–3.4 (b) | 30 (c,d) |
| 18 | Si-NH-CD | Si-Cl, 6-amino β-CD, DMF, 60 °C | 12 h | 4.02 (a)–0.99 (b) | 35 (c) |
| 19 | Si-NH-CD | Si-Cl, 6-amino β-CD, H2O, 60 °C | 12 h | 2.6 (a)–0.81 (b) | 20 (c) |
| 20 | Si-NH-CD | Si-Cl, 6-amino β-CD, DMF, US, 80 kHz, | 2 h | 3.81 (a)–0.68 (b) | 30 (c) |
| 21 | Si-NH-CD | Si-Cl, 6-amino β-CD, H2O, US, 80 kHz, | 2 h | 7.12 (a)–0.60 (b) | 60 (c) |
| 22 | Si-NH-CD | Si-Cl, 6-amino β-CD, DMF, US, 80 kHz, | 4 h | 3.5 (a)–0.67 (b) | 30 (c) |
| 23 | Si-NH-CD | Si-Cl, 6-amino β-CD, H2O, US, 80 kHz, | 4 h | 6.8 (a)–0.68 (b) | 60 (d) |
| Entry | Catalyst, Cu mol% | Yield (%) (a) |
|---|---|---|
| 1 | Cu(OAc)2, 5 mol% | 14 |
| 2 | CuSO4, 5 mol% | 0 |
| 3 | β-CD-CuSO4, 5 mol% (physical mixture) | 0 |
| 4 | β-CD-Cu, 5 mol% (complex) | 55 |
| 5 | Si-NHCD-Cu, 4 mol%, (17 mg) | 5 |
| 6 | Si-DETA-CD-Cu, 4 mol% (24 mg) | >99 |
| 7 | Si-DETA-CD-Cu, 2 mol% (12 mg) | >99 |
| 8 | Si-DETA-CD-Cu, 1 mol% (6 mg) | >99 |
| 9 | Si-DETA-CD-Cu, 0.5 mol% (3 mg) | 80 |
| 10 | Si-DiAm-CD, 4 mol% (11 mg) | >99 |
| 11 | Si-DiAm-CD, 2 mol% (5.5 mg) | 65 |
| 12 | Silica-Cu (12 mg) | 4 |
| 13 | Si-DETA-Cu (12 mg) a | 0 |
| Entry | Product | Reaction Condition | Time | Loading (w/w %) | Loading (μmol/g) |
|---|---|---|---|---|---|
| 1 | Si-diAm | Silica, AEPS, toluene, 80 °C | 6 h | 6.5 (a) | 290 (c) |
| 2 | Si-diAm | Silica, AEPS, toluene, 80 °C | 36 h | 13.6 (a) | 610 (c) |
| 3 | Si-diAm | Silica, AEPS, toluene, US, 40 kHz | 2 h | 14 (a) | 640 (c) |
| 4 | Si-diAm | Silica, AEPS, toluene, US, 80 kHz | 2 h | 12 (a) | 540 (c) |
| 5 | Si-diAm | Silica, AEPS, toluene, US, 80 kHz | 4 h | 13 (a) | 600 (c) |
| 6 | Si-diAm-CD | Si-diAm, 6I-tosyl-β-CD, DMF, 60 °C | 60 h | 4.7 (a)–2.15 (b) | 42 (c) |
| 7 | Si-diAm-CD | Si-diAm, 6I-tosyl-β-CD, DMF, 60 °C | 6 days | 6.1 (a)–2.8 (b) | 54 (c) |
| 8 | Si-diAm-CD | Si-diAm, 6I-tosyl-β-CD, DMF, US, 80 kHz | 4 h | 1.7 (a)–1.18 (b) | 15 (c) |
| 9 | Si-diAm-CD | Si-diAm, 6I-tosyl-β-CD, DMF, MW/US, 100 °C | 4 h | 6.1 (a)–2.09 (b) | 54 (c) |
| Entry | Alkyne | Azide | Product | Yield (%) |
|---|---|---|---|---|
 |  |  | 99 (a) | |
 |  |  | 95 (a) | |
 |  |  | 92 (a) | |
 |  |  | 89 (a) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martina, K.; Calsolaro, F.; Zuliani, A.; Berlier, G.; Chávez-Rivas, F.; Moran, M.J.; Luque, R.; Cravotto, G. Sonochemically-Promoted Preparation of Silica-Anchored Cyclodextrin Derivatives for Efficient Copper Catalysis. Molecules 2019, 24, 2490. https://doi.org/10.3390/molecules24132490
Martina K, Calsolaro F, Zuliani A, Berlier G, Chávez-Rivas F, Moran MJ, Luque R, Cravotto G. Sonochemically-Promoted Preparation of Silica-Anchored Cyclodextrin Derivatives for Efficient Copper Catalysis. Molecules. 2019; 24(13):2490. https://doi.org/10.3390/molecules24132490
Chicago/Turabian StyleMartina, Katia, Federica Calsolaro, Alessio Zuliani, Gloria Berlier, Fernando Chávez-Rivas, Maria Jesus Moran, Rafael Luque, and Giancarlo Cravotto. 2019. "Sonochemically-Promoted Preparation of Silica-Anchored Cyclodextrin Derivatives for Efficient Copper Catalysis" Molecules 24, no. 13: 2490. https://doi.org/10.3390/molecules24132490
APA StyleMartina, K., Calsolaro, F., Zuliani, A., Berlier, G., Chávez-Rivas, F., Moran, M. J., Luque, R., & Cravotto, G. (2019). Sonochemically-Promoted Preparation of Silica-Anchored Cyclodextrin Derivatives for Efficient Copper Catalysis. Molecules, 24(13), 2490. https://doi.org/10.3390/molecules24132490