Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage
Abstract
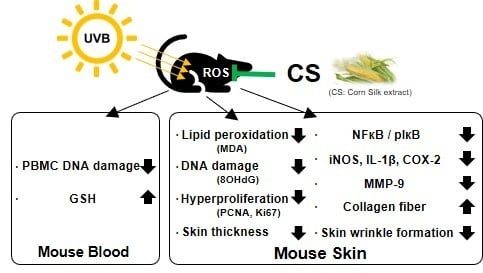
:1. Introduction
2. Results
2.1. DPPH and ABTS Antioxidant Capacities of CS Extract
2.2. Food Intake, Body Weight, and Organ Weights of Animals
2.3. Effects of CS Extract on Skinfold, Epidermal Thickness, and Wrinkle Formation in UVB-Irradiated Mice
2.4. Effect of CS Extract on Epidermal Expression Levels of PCNA and Ki67 in UVB-Irradiated Mice
2.5. Effect of CS Extract on Skin Collagen Fiber Content in UVB-Irradiated Mice
2.6. Effect of CS Extract on Oxidative Stress and Skin Antioxidation Genes
2.7. Effect of CS Extract on Skin Inflammatory Gene Expression in UVB-Irradiated Mice
2.8. Effect of CS Extract on UVB-Irradiated Human Keratinocytes
2.9. Metabolite Identification in CS Using LC-MS/MS
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of CS Extract
4.3. Measurement of Antioxidant Effects of CS Extract
4.4. Experimental Animals
4.5. Assessment of Skin Thickness and Wrinkle Formation in Mice
4.6. Histological and Immunohistochemical Analysis in Mice
4.7. Measurement of Reduced GSH Content in Mouse Plasma
4.8. Comet Assay (Alkaline Single-Cell Gel Electrophoresis)
4.9. Determination of Lipid Peroxidation in Skin Tissue
4.10. Cell Culture
4.11. Cell Viability (MTT) Assay
4.12. Western Blot Analysis
4.13. Reverse Transcriptase (RT) and Quantitative Polymerase Chain Reaction (qPCR)
4.14. Sample Preparation for Liquid Chromatography-Tandem Mass Spectrometer (LC-MS/MS) Analysis
4.15. LC-MS/MS Analysis and Identification of Metabolites
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. Med Fac. Univ. Palackyolomoucczechoslovakia 2003, 147, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Am. J. Pathol. 2004, 165, 741–751. [Google Scholar] [CrossRef]
- Fernández-García, E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014, 5, 1994–2003. [Google Scholar] [CrossRef]
- Ren, S.C.; Liu, Z.L.; Ding, X.L. Isolation and identification of two novel flavone glycosides from corn silk (Stigma maydis). J. Med. Plants Res. 2009, 3, 1009–1015. [Google Scholar]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef]
- Ku, K.M.; Kim, S.K.; Kang, Y.H. Antioxidant activity and functional components of corn silk (Zea mays L.). Korean J. Plant Resour. 2009, 22, 323–329. [Google Scholar]
- Choi, S.Y.; Lee, Y.; Kim, S.S.; Ju, H.M.; Baek, J.H.; Park, C.S.; Lee, D.H. Inhibitory Effect of Corn Silk on Skin Pigmentation. Molecules 2014, 19, 2808–2818. [Google Scholar] [CrossRef] [Green Version]
- Fossen, T.; Slimestad, R.; Andersen, Ø.M. Anthocyanins from maize (Zea mays) and reed canarygrass (Phalaris arundinacea). J. Agric. Food Chem. 2001, 49, 2318–2321. [Google Scholar] [CrossRef]
- Hu, Q.; Deng, Z. Protective effects of flavonoids from corn silk on oxidative stress induced by exhaustive exercise in mice. Afr. J. Biotechnol. 2011, 10, 3163–3167. [Google Scholar] [Green Version]
- Maksimović, Z.; Malenčić, Đ.; Kovačević, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Wang, Z.; Zhang, C.; Lu, S.; Liu, J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011, 126, 261–269. [Google Scholar] [CrossRef]
- Wang, K.-J.; Zhao, J.-L. Corn silk (Zea mays L.), a source of natural antioxidants with α-amylase, α-glucosidase, advanced glycation and diabetic nephropathy inhibitory activities. Biomed. Pharmacother. 2019, 110, 510–517. [Google Scholar] [CrossRef]
- Tian, J.; Chen, H.; Chen, S.; Xing, L.; Wang, Y.; Wang, J. Comparative studies on the constituents, antioxidant and anticancer activities of extracts from different varieties of corn silk. Food Funct. 2013, 4, 1526–1534. [Google Scholar] [CrossRef]
- Choi, D.J.; Kim, S.-L.; Choi, J.W.; Park, Y.I. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014, 109, 57–64. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yuan, W.; Roan, H.-Y.; Chang, J.-L.; Huang, H.-C.; Lee, Y.-C.; Tsay, H.J.; Liu, H.-K. The ethyl acetate fraction of corn silk exhibits dual antioxidant and anti-glycation activities and protects insulin-secreting cells from glucotoxicity. Bmc Complementary Altern. Med. 2016, 16, 432. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, L.; Chen, Y.; Ma, Q.; Santhanam, R.K.; Xue, Z.; Gao, X.; Chen, H. Structural characterization of corn silk polysaccharides and its effect in H2O2 induced oxidative damage in L6 skeletal muscle cells. Carbohydr. Polym. 2019, 208, 161–167. [Google Scholar] [CrossRef]
- Vranješ, M.; Popović, B.M.; Štajner, D.; Ivetić, V.; Mandić, A.; Vranješ, D. Effects of bearberry, parsley and corn silk extracts on diuresis, electrolytes composition, antioxidant capacity and histopathological features in mice kidneys. J. Funct. Foods 2016, 21, 272–282. [Google Scholar] [CrossRef]
- Oyabambi, A.O.; Areola, E.D.; Olatunji, L.A.; Soladoye, A.O. Uric acid is a key player in salt-induced endothelial dysfunction: The therapeutic role of stigma maydis (corn silk) extract. Appl. Physiol. Nutr. Metab. 2019. [Google Scholar] [CrossRef]
- Bai, H.; Hai, C.; Xi, M.; Liang, X.; Liu, R. Protective Effect of Maize Silks (Maydis stigma) Ethanol Extract on Radiation-Induced Oxidative Stress in Mice. Plant Foods Hum. Nutr. 2010, 65, 271–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Ma, Z.; Cheng, J.; Liu, J. Anti-Diabetic, Anti-Oxidant and Anti-Hyperlipidemic Activities of Flavonoids from Corn Silk on STZ-Induced Diabetic Mice. Molecules 2016, 21, 7. [Google Scholar] [CrossRef]
- Yang, B.; Ji, C.; Kang, J.; Chen, W.; Bi, Z.; Wan, Y. Trans-Zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. Int. J. Mol. Med. 2009, 23, 555–560. [Google Scholar] [Green Version]
- Byun, S.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Hwang, M.K.; Lim, S.H.; Bode, A.M.; Lee, H.J.; Dong, Z. Luteolin inhibits protein kinase Cε and c-Src activities and UVB-induced skin cancer. Cancer Res. 2010, 70, 2415–2423. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. New Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Palei, A.C.; Sandrim, V.C.; Cavalli, R.C.; Tanus-Santos, J.E. Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin. Biochem. 2008, 41, 875–880. [Google Scholar] [CrossRef]
- Carrara, I.M.; Melo, G.P.; Bernardes, S.S.; Neto, F.S.; Ramalho, L.N.Z.; Marinello, P.C.; Luiz, R.C.; Cecchini, R.; Cecchini, A.L. Looking beyond the skin: Cutaneous and systemic oxidative stress in UVB-induced squamous cell carcinoma in hairless mice. J. Photochem. Photobiol. B: Biol. 2019, 195, 17–26. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafezi, S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef]
- Svobodova, A.R.; Galandáková, A.; Šianská, J.; Doležal, D.; Ulrichová, J.; Vostálová, J. Acute exposure to solar simulated ultraviolet radiation affects oxidative stress-related biomarkers in skin, liver and blood of hairless mice. Biol. Pharm. Bull. 2011, 34, 471–479. [Google Scholar] [CrossRef]
- Barg, M.; Rezin, G.T.; Leffa, D.D.; Balbinot, F.; Gomes, L.M.; Carvalho-Silva, M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Streck, E.L. Evaluation of the protective effect of Ilex paraguariensis and Camellia sinensis extracts on the prevention of oxidative damage caused by ultraviolet radiation. Environ. Toxicol. Pharmacol. 2014, 37, 195–201. [Google Scholar] [CrossRef]
- Sathishsekar, D.; Subramanian, S. Beneficial effects of Momordica charantia seeds in the treatment of STZ-induced diabetes in experimental rats. Biol. Pharm. Bull. 2005, 28, 978–983. [Google Scholar] [CrossRef]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 12475. [Google Scholar] [CrossRef]
- Kawachi, Y.; Xu, X.; Taguchi, S.; Sakurai, H.; Nakamura, Y.; Ishii, Y.; Fujisawa, Y.; Furuta, J.; Takahashi, T.; Itoh, K. Attenuation of UVB-induced sunburn reaction and oxidative DNA damage with no alterations in UVB-induced skin carcinogenesis in Nrf2 gene-deficient mice. J. Investig. Dermatol. 2008, 128, 1773–1779. [Google Scholar] [CrossRef]
- Hirota, A.; Kawachi, Y.; Yamamoto, M.; Koga, T.; Hamada, K.; Otsuka, F. Acceleration of UVB-induced photoageing in nrf2 gene-deficient mice. Exp. Dermatol. 2011, 20, 664–668. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2009, 83, 354–361. [Google Scholar] [CrossRef]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. Febs Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Jia, Z.; Zhang, L.; Yamamoto, M.; Misra, H.P.; Trush, M.A.; Li, Y. Antioxidants and phase 2 enzymes in macrophages: Regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp. Biol. Med. (Maywoodn.J.) 2008, 233, 463–474. [Google Scholar] [CrossRef]
- Liu, X.; Jann, J.; Xavier, C.; Wu, H. Glutaredoxin 1 (Grx1) protects human retinal pigment epithelial cells from oxidative damage by preventing AKT glutathionylation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2821–2832. [Google Scholar] [CrossRef]
- Batliwala, S.; Xavier, C.; Liu, Y.; Wu, H.; Pang, I.-H. Involvement of Nrf2 in ocular diseases. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Heo, H.S.; Han, G.E.; Won, J.; Cho, Y.; Woo, H.; Lee, J.H. Pueraria montana var. lobata root extract inhibits photoaging on skin through Nrf2 pathway. J. Microbiol. Biotechnol. 2019, 29, 518–526. [Google Scholar] [CrossRef]
- Kannan, S.; Jaiswal, A.K. Low and high dose UVB regulation of transcription factor NF-E2-related factor 2. Cancer Res. 2006, 66, 8421–8429. [Google Scholar] [CrossRef]
- Chang, E.J.; Kundu, J.K.; Liu, L.; Shin, J.W.; Surh, Y.J. Ultraviolet B radiation activates NF-kappaB and induces iNOS expression in HR-1 hairless mouse skin: Role of IkappaB kinase-beta. Mol. Carcinog. 2011, 50, 310–317. [Google Scholar] [CrossRef]
- Abeyama, K.; Eng, W.; Jester, J.V.; Vink, A.A.; Edelbaum, D.; Cockerell, C.J.; Bergstresser, P.R.; Takashima, A. A role for NF-kappaB-dependent gene transactivation in sunburn. J. Clin. Investig. 2000, 105, 1751–1759. [Google Scholar] [CrossRef]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol. Cancer Ther. 2007, 6, 995–1005. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef]
- Nausheen, J.; Sabina Bibi Jhaumeer, L.; Prakashanand, C.; Prashant Suresh, K. Antioxidant, Antidiabetic and Anticancer Activities of L-Phenylalanine and L-Tyrosine Ester Surfactants: In vitro and In Silico Studies of their Interactions with Macromolecules as Plausible Mode of Action for their Biological Properties. Curr. Bioact. Compd. 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Saxena, R.; Pendse, V.; Khanna, N. Anti-inflammatory and analgesic properties of four amino-acids. Indian J. Physiol. Pharmacol. 1984, 28, 299–305. [Google Scholar]
- Pavicic, T.; Wollenweber, U.; Farwick, M.; Korting, H. Anti-microbial and-inflammatory activity and efficacy of phytosphingosine: An in vitro and in vivo study addressing acne vulgaris. Int. J. Cosmet. Sci. 2007, 29, 181–190. [Google Scholar] [CrossRef]
- Kwon, S.B.; An, S.; Kim, M.J.; Kim, K.R.; Choi, Y.M.; Ahn, K.J.; An, I.-S.; Cha, H.J. Phytosphingosine-1-phosphate and epidermal growth factor synergistically restore extracellular matrix in human dermal fibroblasts in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Digby, J.E.; McNeill, E.; Dyar, O.J.; Lam, V.; Greaves, D.R.; Choudhury, R.P. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis 2010, 209, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Pullar, J.; Carr, A.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Marini, A. Beauty from the inside. Does it really work? Der Hautarzt; Z. Fur Dermatol. Venerol. Und Verwandte Geb. 2011, 62, 614–617. [Google Scholar] [CrossRef]
- Reifen, R. Vitamin A as an anti-inflammatory agent. Proc. Nutr. Soc. 2002, 61, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Khanpour, E.; Modarresi, M. Quantitative analysis of allantoin in Iranian corn silk. Res. J. Pharmacogn. 2017, 4, 16. [Google Scholar]
- Žilić, S.; Janković, M.; Basić, Z.; Vančetović, J.; Maksimović, V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): Comparison with medicinal herbs. J. Cereal Sci. 2016, 69, 363–370. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, N.H.; Jung, D.; Lee, J.A.; Seo, C.S.; Lee, H.; Kim, J.H.; Shin, H.K. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int. Immunopharmacol. 2010, 10, 474–480. [Google Scholar] [CrossRef]
- Cunniff, P.; Association of Official Agricultural, C. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MA, USA, 1996. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Guo, J.; Liu, T.; Han, L.; Liu, Y. The effects of corn silk on glycaemic metabolism. Nutr Metab (Lond) 2009, 6, 47. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Liu, J.; Lu, S.; Zhang, C.; Wang, E.; Wang, Z.; Zhang, Y.; Liu, J. Subchronic toxicity study of corn silk with rats. J. Ethnopharmacol. 2011, 137, 36–43. [Google Scholar] [CrossRef]
- Mantena, S.K.; Meeran, S.M.; Elmets, C.A.; Katiyar, S.K. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J. Nutr. 2005, 135, 2871–2877. [Google Scholar] [CrossRef]
- Record, I.R.; Dreosti, I.E. Protection by black tea and green tea against UVB and UVA+B induced skin cancer in hairless mice. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 1998, 422, 191–199. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |







| Sample | IC50 (mg/mL) | |
|---|---|---|
| DPPH | ABTS | |
| Corn silk extract | 3.60 ± 0.1 | 11.61 ± 0.2 |
| L-ascorbic acid | 0.08 ± 0.0 | 0.38 ± 0.1 |
| Gene | Forward Sequence | Reverse Sequence | Product Size |
|---|---|---|---|
| m.Catalase | AACGCTGGATGGATTCTCCC | GCCCTAACCTTTCATTTCCCTTCAG | 133 |
| m.Procollagen type 1 | CCCTAGCCTTTTCTCCGCC | TGGCAACTCCAAGTCCATCAT | 238 |
| m.Nrf2 | GTGAGACGTGGAAACCCGAG | GCCATAGGACATCTGGGAAGC | 347 |
| m.TXN | GAGCAAGGAAGCTTTTCAGGAG | GTCCCGTTTTGGATCCGAGT | 252 |
| m.SOD1 | ATGGCGACGAAGGCCGTGTG | GACCACCAGTGTGCGGCCAA | 360 |
| m.GAPDH | AAGGTCGGTGTGAACGGATTT | CAGAAGGGGCGGAGATGATG | 364 |
| h.Glutaredoxin | CATCGGCATGGCTCAAGAG | AATCTGCTTTAGCCGCGTCA | 313 |
| h.Procollagen type 1 | AGGACAAGAGGCATGTCTGGTT | TTGCAGTGTAGGTGATGTTCTG | 156 |
| h.GAPDH | AAGGTCGGTGTGAACGGATTT | CAGAAGGGGCGGAGATGATG | 364 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-h.; Cho, A.; Kwon, S.-A.; Kim, M.; Song, M.; Han, H.w.; Shin, E.-J.; Park, E.; Lee, S.-M. Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage. Molecules 2019, 24, 2587. https://doi.org/10.3390/molecules24142587
Kim Y-h, Cho A, Kwon S-A, Kim M, Song M, Han Hw, Shin E-J, Park E, Lee S-M. Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage. Molecules. 2019; 24(14):2587. https://doi.org/10.3390/molecules24142587
Chicago/Turabian StyleKim, Yeon-hee, Amy Cho, Sang-Ah Kwon, Minju Kim, Mina Song, Hye won Han, Eun-Ji Shin, Eunju Park, and Seung-Min Lee. 2019. "Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage" Molecules 24, no. 14: 2587. https://doi.org/10.3390/molecules24142587
APA StyleKim, Y. -h., Cho, A., Kwon, S. -A., Kim, M., Song, M., Han, H. w., Shin, E. -J., Park, E., & Lee, S. -M. (2019). Potential Photoprotective Effect of Dietary Corn Silk Extract on Ultraviolet B-Induced Skin Damage. Molecules, 24(14), 2587. https://doi.org/10.3390/molecules24142587