Polyphenolic and Physicochemical Properties of Simple-Spined Num-Num (Carissa edulis) Fruit Harvested at Ripe Stage of Maturation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Carissa edulis Fruit Samples
2.1.1. pH and Total Titratable Acidity (TTA) of Carissa edulis Fruit
2.1.2. Total Soluble Solids of Carissa edulis Fruit
2.1.3. Sugar/Acid Ratio of Carissa edulis Fruit
2.1.4. Colour Properties of Carissa edulis Fruit
2.2. Phytochemical Properties of Carissa edulis Fruit Samples
2.2.1. Total Phenolic Content (TPC) of Carissa edulis Fruit
2.2.2. Total Flavonoids of Carissa edulis Fruit
2.2.3. 2,2 diphenyl-1-picryl-hydrazyl (DPPH) Radical Scavenging Activity of Carissa edulis Fruit
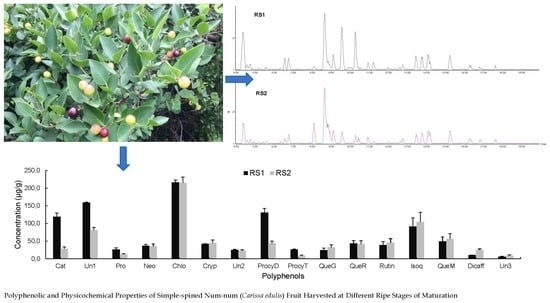
2.2.4. Polyphenolic Profile of Carissa edulis Fruit
3. Materials and Method
3.1. Fruit Samples
3.2. Preparation of Plant Extracts
3.3. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
3.4. Total Phenolic Content Determination
3.5. Total Flavonoids Determination
3.6. Determination of pH and Titratable Acidity
3.7. Determination of Soluble Solids (oBrix)
3.8. Determination of Colour Parameters
3.9. Liquid Chromatography Coupled to Diode Array Detection and Electrospray Ionisation Mass Spectrometry (LC-DAD-ESI-MS) Analysis of Phenolic Compounds in Carissa edulis Fruit
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforestry database: A tree species reference and selection guide version 4.0. Available online: https://www.worldagroforestry.org (accessed on 27 April 2017).
- Mutshinyalo, T.; Malatji, R. Carissa edulis. Available online: Pza.Sanbi.Org/Carissa-edulis (accessed on 15 August 2017).
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Makumbele, F.P. Assessment of physicochemical, polyphenolic and antioxidant properties in Carissa edulis fruit harvested at ripe stage of maturation. Honours Thesis, University of Venda, Thohoyandou, South Africa, 23 March 2018. [Google Scholar]
- Namiesnik, J.; Vearasilp, K.; Nemirovski, A.; Leontowicz, H.; Leontowicz, M.; Pasko, P.; Martinez-Ayala, A.L.; González-Aguilar, G.A.; Suhaj, M.; Gorinstein, S. In vitro studies on the relationship between the antioxidant activities of some berry extracts and their binding properties to serum albumin. Appl. Biochem. Biotech. 2014, 172, 2849–2865. [Google Scholar] [CrossRef]
- Hamid, H.; Yousef, H.; Jafar, H.; Mohammad, A. Antioxidant capacity and phytochemical properties of cornelian cherry (Cornus mas L.) genotypes in Iran. Sci. Hortic. 2011, 129, 459–463. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Green, R.C. Physicochemical properties and phenolic composition of selected Saskatchewan fruits: Buffaloberry, chokecherry and sea buckthorn. Doctoral Dissertation, University of Saskatchewan, Saskatoon, SK, Canada, 2007; pp. 11–300. Available online: http://hdl.handle.net/10388/etd-07262007-084516 (accessed on 23 November 2017).
- Bagchi, D.; Sen, C.K.; Bagchi, M.; Atalay, M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. J. Biochem. 2004, 69, 75–80. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmianski, J.; Pluta, S. The composition of bioactive compounds and antioxidant activity of Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in central Poland. Food Chem. 2017, 235, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Rampedi, I.T.; Olivier, J. Traditional beverages derived from wild food plant species in the Vhembe District, Limpopo Province in South Africa. Ecol. Food Nutr. 2013, 52, 203–222. [Google Scholar] [CrossRef]
- Nandal, U.; Bhardwaj, R. The role of underutilized fruits in nutritional and economic security of tribals: A review. Crit. Rev. Food Sci. 2014, 54, 880–890. [Google Scholar] [CrossRef]
- Elfiky, F.K.; Aboukaram, M.A.; Afify, E.A. Effect of Luffa aegyptiaca (seeds) and Carissa edulis (leaves) extracts on blood glucose level of normal and streptozotocin diabetic rats. J. Ethnopharmacol. 1996, 50, 43–47. [Google Scholar] [CrossRef]
- Kirira, P.G.; Rukunga, G.M.; Wanyonyi, A.W.; Muregi, F.M.; Gathirwa, J.W.; Muthaura, C.N.; Omar, S.A.; Tolo, F.; Mungai, G.M.; Ndiege, I.O. Anti-plasmodial activity and toxicity of extracts of plants used in traditional malaria therapy in Meru and Kilifi Districts of Kenya. J. Ethnopharmacol. 2006, 106, 403–407. [Google Scholar] [CrossRef]
- Moudachirou, M.; Ayedoun, M.A.; Gbenou, J.D.; Garneau, F.-X.; Gagnon, H.; Jean, F.-I. Essential Oil of Carissa edulis Vahl. from Benin. J. Essent. Oil Res. 1998, 10, 195–196. [Google Scholar] [CrossRef]
- Ya’u, J.; Yaro, A.H.; Abubakar, M.S. Anticonvulsant activity of Carissa edulis (Vahl) (Apocynaceae) root bark extract. J. Ethnopharmacol. 2008, 120, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Bagirova, M.; Yaman, S. Development of New Antiherpetic Drugs Based on Plant Compounds. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M., Kon, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 245–259. [Google Scholar]
- Tolo, F.M.; Rukunga, G.M.; Muli, F.W. Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J. Ethnopharmacol. 2006, 104, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Bagla, V.P.; McGaw, L.J.; Eloff, J.N. The antiviral activity of six South African plants traditionally used against infections in ethnoveterinary medicine. Vet. Microbiol. 2012, 155, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrier, N.; Sauvage, F.X.; Ageorges, A.; Romieu, C. Changes in acidity and in proton transport at the tonoplast of grape berries during development. Planta 2001, 213, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.L. Cell-wall Metabolism and Softening during Ripening. In Fruit ripening - Physiology, Signalling and Genomics; Nath, P., Bouzayen, M., Mattoo, A.K., Claude Pech, J., Eds.; CABI International: Wallingford, Oxfordshire, UK, 2014; pp. 48–62. [Google Scholar]
- Zarei, M.; Azizi, M.; Bashir-Sadr, Z. Evaluation of physicochemical characteristics of pomegranate (Punica granatum L.) fruit during ripening. Fruits 2011, 66, 121–129. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Viskelis, P.; Jasutiene, I.; Duchovskis, P.; Bobinas, C. Changes in biologically active constituents during ripening in black currants. J. Fruit Ornament. Plant Res. 2006, 14, 237. [Google Scholar]
- Robinson, J.; Harding, J. The Oxford companion to wine, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.G.; Hafiz, I.A.; Chaudhary, A.H.; Khan, M.I.; Chaudhary, M.I. Quality estimation during marketing of kinnow and feutrell’s early. Int. J. Agric. Biol. 2000, 2, 328–330. [Google Scholar]
- Gunduz, K.; Saracoglu, O.; Özgen, M.; Serce, S. Antioxidant, physical and chemical characteristics of cornelian cherry fruits (Cornus mas L.) at different stages of ripeness. Acta Sci. Pol-Hortoru. 2013, 12, 59–66. [Google Scholar]
- Celik, S.; Bakırcı, I.; Şat, I.G. Physicochemical and organoleptic properties of yogurt with cornelian cherry paste. Int. J. Food Prop. 2006, 9, 401–408. [Google Scholar] [CrossRef]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Eichholz, I.; Huyskens-Keil, S.; Kroh, L.W.; Rohn, S. Phenolic compounds, pectin and antioxidant activity in blueberries (Vaccinium corymbosum L.) influenced by boron and mulch cover. J. Appl. Bot. Food Qual. 2011, 84, 26–32. [Google Scholar]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena–an overview. Crit. Rev. Food Sci. 2007, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Kalt, W.; Lawand, C.; Ryan, D.A.; McDonald, J.E.; Donner, H.; Forney, C.F. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Mäkilä, L. Effect of Processing Technologies on Phenolic Compounds in Berry Products. Ph.D. Thesis, The University of Turku, Turku, Finland, 15 May 2017. [Google Scholar]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H. Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Shin, Y.; Ryu, J.A.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Tec. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Erba, D.; Casiraghi, M.C.; Ribas-Agustí, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, colour attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J. Anal. Methods Chem. 2016, 1–13. [Google Scholar] [CrossRef]
- Kutz, M. Handbook of Environmental Degradation of Materials; Norwich: New York, NY, USA, 2005; pp. 465–503. [Google Scholar]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colourful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Eichholz, I.; Kroh, L.W.; Rohn, S. UV-B induced changes of phenol composition and antioxidant activity in black currant fruit (Ribes nigrum L.). J. Appl. Bot. Food Qual. 2007, 81, 140–144. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Yanishlieva-Maslarova, N.V.; Heinonen, I.M. Sources of natural antioxidants: Vegetables, fruits, herbs, spices and teas. In Antioxidants in Food: Practical Applications; Pokorny, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 210–263. [Google Scholar]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007, 100, 1409–1418. [Google Scholar] [CrossRef]
- Jaakola, L.; Maattä, K.; Pirttila, A.M.; Torronen, R.; Karenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef]
- Halbwirth, H.; Puhl, I.; Hass, U.; Jezik, K.; Treutter, D.; Stich, K. Two-phase flavonoid formation in developing strawberry (Fragaria ananassa) fruit. J. Agric. Food Chem. 2006, 54, 1479–1485. [Google Scholar] [CrossRef]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.; Rupasinghe, H.P.; Forney, C.F.; Eaton, L. Characterisation of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef]
- Juríková, T.; Balla, S.; Sochor, J.; Pohanka, M.; Mlcek, J.; Baron, M. Flavonoid Profile of Saskatoon Berries (Amelanchier alnifolia Nutt.) and Their Health Promoting Effects. Molecules 2013, 18, 12571–12586. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-coloured phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef]
- Stanila, A.; Diaconeasa, Z.; Roman, I.; Nicusor, S.I.M.A.; Maniutiu, D.; Roman, A.; Rodica, S.I.M.A. Extraction and Characterisation of Phenolic Compounds from Rose Hip. Not. Bot. Horti. Agrobo. 2015, 43, 349. [Google Scholar]
- Hithamani, G.; Srinivasan, K. Bioaccessibility of polyphenols from wheat (Triticum aestivum), sorghum (Sorghum bicolor), green gram (Vigna radiata), and chickpea (Cicer arietinum) as influenced by domestic food processing. J. Agr. Food Chem. 2014, 62, 11170–11179. [Google Scholar] [CrossRef]
- De Ancos, B.; Sgroppo, S.; Plaza, L.; Cano, M.P. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J. Sci. Food Agric. 2002, 82, 790–796. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- AOAC International. The Official Method of Analysis, 15th ed.; The Association Official Analytical Chemist International: Arlington, VA, USA, 1990. [Google Scholar]
Sample Availability: Not available. |






| Samples | pH | TTA (g/100 mL) | TSS (oBrix) | TSS/TTA |
|---|---|---|---|---|
| RS1 | 2.85 ± 0.04 a | 0.37 ± 0.01 c | 9.51 ± 0.21 a | 26.03 |
| RS2 | 2.91 ± 0.01 a | 0.35 ± 0.01 c | 11.12 ± 0.08 b | 31.95 |
| RS3 | 3.10 ± 0.01 b | 0.27 ± 0.02 b | 13.51 ± 0.21 d | 50.36 |
| SR | 3.32 ± 0.07 c | 0.21 ± 0.02 a | 13.14 ± 0.07 c | 61.55 |
| Samples | TPC (mg GAE/g) | TFC (mg CE/g) | DPPH (mmol TE/g) |
|---|---|---|---|
| RS1 | 6.81 ± 0.02 b | 5.09 ± 0.04 b | 20.24 ± 0.27 c |
| RS2 | 5.90 ± 0.41 a | 5.92 ± 0.03 b | 19.09 ± 0.02 b |
| RS3 | 6.71 ± 0.13 c | 5.95 ± 0.76 a | 18.36 ± 0.12 a |
| SR | 7.21 ± 0.23 c | 6.31 ± 0.27 b | 20.26 ± 0.56 c |
| Peak No. | Rt (min) | [M − H]− (m/z) | [M − H]− Formula | Error (ppm) | MSE Fragments (m/z) | UV (nm) | Tentative Identification | Classification |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.16 | 191.0539 | C7H11O6 | 2.6 | 85 | 264 | Quinic acid | Phenolic acids (Cyclic polyol) |
| 2 | 2.17 | 191.0181 | C6H7O7 | 0.6 | 155, 127, 111 | 280 | Citric acid | Organic acid |
| 3 | 4.35 | 309.1188 | C12H22O9 | −1.0 | 309, 129 | Weak | Unknown | |
| 4 | 6.56 | 315.0715 | C13H15O9 | −0.3 | 153, 109 | 306 | Protocatechuoyl-hexose | Phenolic acids |
| 5 | 6.75 | 353.0854 | C16H17O9 | 1.7 | 191, 179, 135 | 325 | Neochlorogenic acid (3CQA) | Phenolic acids |
| 6 | 8.09 | 175.0605 | C7H11O5 | −2.9 | 115 | Weak | Unknown | |
| 7 | 8.65 | 353.0876 | C16H17O9 | −2.5 | 191 | 325 | Chlorogenic acid (5CQA) | Phenolic acids |
| 8 | 9.10 | 353.0886 | C16H17O9 | 0.8 | 191, 179, 173, 135 | 325 | Cryptochlorogenic acid (4CQA) | Phenolic acids |
| 9 | 9.56 | 577.1357 | C30H25O12 | 2.6 | 407, 289 | 279 | Procyanidin dimer | Flavonoid (Proanthocyanidin/condensed tannin) |
| 10 | 10.26 | 289.0695 | C15H13O6 | 1.7 | 245, 203, 179, 137, 125 | 278 | Catechin | Flavonoid (Flavan-3-ol) |
| 11 | 10.93 | 439.1859 | C18H31O12 | −1.8 | 408, 289, 161, 125 | 278 | Unknown | |
| 12 | 11.21 | 865.1946 | C45H37O18 | −3.9 | 695, 575, 407, 289, 161 | 278 | Procyanidin trimer | Flavonoid (Proanthocyanidin/condensed tannin) |
| 13 | 12.75 | 595.132 | C26H27O16 | 3.5 | 300, 271, 255 | 349 | Quercetin-3-O-glucosyl-xyloside | Flavonoid (Flavonol-glycoside) |
| 14 | 13.44 | 609.1467 | C27H29O16 | 3.8 | 301, 300, 271, 255 | 351 | Quercetin-3-O-robinobioside | Flavonoid (Flavonol-glycoside) |
| 15 | 13.75 | 609.147 | C27H29O16 | 2.6 | 301, 300, 271, 255 | 351 | Quercetin-3-O-rutinoside (rutin) | Flavonoid (Flavonol-glycoside) |
| 16 | 14.06 | 463.0867 | C21H19O12 | 0.4 | 300, 271, 255 | 353 | Quercetin-3-O-glucoside (isoquercitrin) | Flavonoid (Flavonol-glycoside) |
| 17 | 15.24 | 607.1327 | C27H27O16 | −0.5 | 505, 463, 300, 271 | 351 | Quercetin-3-OH-3-methylglutaryl-glucoside | Flavonoid (Flavonol-glycoside) |
| 18 | 16.82 | 515.1204 | C25H23O12 | 2.7 | 191, 179, 173, 135 | 329 | Dicaffeoylquinic acid | Phenolic acids |
| 19 | 17.61 | 1381.4824 | C60H85O36 | −0.3 | 869, 827, 511, 409 | 277 | Unknown |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makumbele, F.P.; Taylor, M.; Stander, M.; Anyasi, T.A.; Jideani, A.I.O. Polyphenolic and Physicochemical Properties of Simple-Spined Num-Num (Carissa edulis) Fruit Harvested at Ripe Stage of Maturation. Molecules 2019, 24, 2630. https://doi.org/10.3390/molecules24142630
Makumbele FP, Taylor M, Stander M, Anyasi TA, Jideani AIO. Polyphenolic and Physicochemical Properties of Simple-Spined Num-Num (Carissa edulis) Fruit Harvested at Ripe Stage of Maturation. Molecules. 2019; 24(14):2630. https://doi.org/10.3390/molecules24142630
Chicago/Turabian StyleMakumbele, Fulufhelo P., Malcolm Taylor, Marietjie Stander, Tonna A. Anyasi, and Afam I.O. Jideani. 2019. "Polyphenolic and Physicochemical Properties of Simple-Spined Num-Num (Carissa edulis) Fruit Harvested at Ripe Stage of Maturation" Molecules 24, no. 14: 2630. https://doi.org/10.3390/molecules24142630
APA StyleMakumbele, F. P., Taylor, M., Stander, M., Anyasi, T. A., & Jideani, A. I. O. (2019). Polyphenolic and Physicochemical Properties of Simple-Spined Num-Num (Carissa edulis) Fruit Harvested at Ripe Stage of Maturation. Molecules, 24(14), 2630. https://doi.org/10.3390/molecules24142630