Research and Development of Zincoborates: Crystal Growth, Structural Chemistry and Physicochemical Properties
Abstract
:1. Introduction
2. Structural Chemistry of Zincoborates
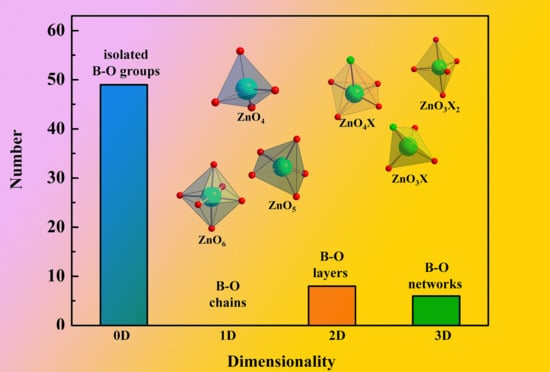
2.1. Statistical Analysis of Structural Configurations
2.2. Zincoborates Possessing Special Structural Features
2.2.1. Zincoborates with Benign KBe2BO3F2 (KBBF)-Type Layered Structures
AZn2BO3X2 (A = Na, K, Rb, NH4; X = Cl, Br) Series with 2∞[Zn2BO3X2] Layers
Cs3Zn6B9O21 with 2∞[Zn2BO3O2] Layers
BaLiZn3(BO3)3 with 2∞[LiZn3(BO3)3] Layers and CdZn2KB2O6F with 2∞[ZnBO3] Layers
2.2.2. Zincoborates with Novel Edge-Sharing [BO4]5− Tetrahedra
KZnB3O6
Ba4Na2Zn4(B3O6)2(B12O24)
2.2.3. Zincoborates with Two Kinds of Isolated Anion Groups
3. Zincoborates with Excellent Properties
3.1. Zincoborates with Short Ultraviolet (UV) Cutoff Edges
3.2. Zincoborates with Large Second-Order Non-Linear Optical (NLO) Response
3.2.1. NLO Properties of Zincoborates Containing Alkali/Alkaline-Earth Metals
Cs3Zn6B9O21
AZn2BO3X2 (A = Na, K, Rb, NH4; X = Cl, Br) Series
BaZnBO3F
Ba5Zn4(BO3)6
3.2.2. Other Zinc-Containing Compounds with NLO Properties
Bi2ZnOB2O6
Ba3(ZnB5O10)PO4
3.3. Zincoborates with Anomalous Thermal Expansion Properties
3.3.1. Near-Zero Thermal Expansion Properties in Zn4B6O13
3.3.2. Unidirectional Thermal Expansion in KZnB3O6
4. Single Crystal Growth of Zincoborates
4.1. Bi2ZnOB2O6
4.2. Ba3(ZnB5O10)PO4
4.3. β-Zn3BPO7
4.4. Zn4B6O13
4.5. BaZnBO3F
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.T.; Liu, G.Z. Recent advances in nonlinear optical and electro-optical materials. Annu. Rev. Mater. Sci. 1986, 16, 203–243. [Google Scholar] [CrossRef]
- Chen, C.T.; Ye, N.; Lin, J.; Jiang, J.; Zeng, W.R.; Wu, B.C. Computer-assisted search for nonlinear optical crystals. Adv. Mater. 1999, 11, 1071–1078. [Google Scholar] [CrossRef]
- Becker, P. Borate materials in nonlinear optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Halasyamani, P.S.; Poeppelmeier, K.R. Noncentrosymmetric oxides. Chem. Mater. 1998, 10, 2753–2769. [Google Scholar] [CrossRef]
- Halasyamani, P.S.; Rondinelli, J.M. The must-have and nice-to-have experimental and computational requirements for functional frequency doubling deep-UV crystals. Nat. Commun. 2018, 9, 2972. [Google Scholar] [CrossRef]
- Ok, K.M.; Chi, E.O.; Halasyamani, P.S. Bulk characterization methods for non-centrosymmetric materials: Second-harmonic generation, piezoelectricity, pyroelectricity, and ferroelectricity. Chem. Soc. Rev. 2006, 35, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Yu, H.W.; Rondinelli, J.M.; Poeppelmeier, K.R.; Halasyamani, P.S. Deep ultraviolet nonlinear optical materials. Chem. Mater. 2016, 28, 5238–5258. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, J.H.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. KSbB2O6 and BaSb2B4O12: Novel boroantimonates with 3D anionic architectures composed of 1D chains of SbO6 octahedra and B2O5 groups. Inorg. Chem. 2014, 53, 3847–3853. [Google Scholar] [CrossRef]
- Yan, D.; Hu, C.L.; Mao, J.G. A2SbB3O8 (A = Na, K, Rb) and β-RbSbB2O6: Two types of alkali boroantimonates with 3D anionic architectures composed of SbO6 octahedra and borate groups. CrystEngComm 2016, 18, 1655–1664. [Google Scholar] [CrossRef]
- Zhang, M.; An, D.H.; Hu, C.; Chen, X.L.; Yang, Z.H.; Pan, S.L. Rational design via synergistic combination leads to an outstanding deep-ultraviolet birefringent Li2Na2B2O5 material with an unvalued B2O5 functional gene. J. Am. Chem. Soc. 2019, 141, 3258–3264. [Google Scholar] [CrossRef]
- Mutailipu, M.; Zhang, M.; Yang, Z.H.; Pan, S.L. Targeting the next generation of deep-ultraviolet nonlinear optical materials: Expanding from borates to borate fluorides to fluorooxoborates. Acc. Chem. Res. 2019, 52, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.F.; Wang, Y.B.; Chen, C.T.; Wu, B.C. Nonlinear optical materials based on MBe2BO3F2 (M = Na, K). J. Appl. Phys. 1993, 74, 7014–7015. [Google Scholar] [CrossRef]
- Mei, L.; Huang, X.; Wang, Y.; Wu, Q.; Wu, B.; Chen, C. Crystal structure of KBe2BO3F2. Z. Kristallogr. 1995, 210, 93–95. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Y.B.; Wu, B.C.; Wu, K.C.; Zeng, W.L.; Yu, L.H. Design and synthesis of an ultraviolet-transparent nonlinear optical crystal Sr2Be2B2O7. Nature 1995, 373, 322–324. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Y.B.; Xia, Y.N.; Wu, B.C.; Tang, D.Y.; Wu, K.C.; Zeng, W.R.; Yu, L.H.; Mei, L.F. New development of nonlinear optical crystals for the ultraviolet region with molecular engineering approach. J. Appl. Phys. 1995, 77, 2268–2272. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, G.M. A new-type ultraviolet SHG crystal–β-BaB2O4. Sci. Sin. B 1985, 28, 235–243. [Google Scholar]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New nonlinear-optical crystal: LiB3O5. J. Opt. Soc. Am. B 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Wu, H.Q.; Ju, P.; He, H.; Yang, B.F.; Yang, G.Y. Three new mixed-alkali- and alkaline-earth-metal borates: From 1D chain to 2D layer to 3D framework. Inorg. Chem. 2013, 52, 10566–10570. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, G.Y. A novel supramolecular magnesoborate framework with snowflake-like channels built by unprecedented huge B69 cluster cages. Chem. Commun. 2017, 53, 10398–10401. [Google Scholar] [CrossRef]
- Kong, F.; Huang, S.P.; Sun, Z.M.; Mao, J.G.; Cheng, W.D. Se2(B2O7): A new type of second-order NLO material. J. Am. Chem. Soc. 2006, 128, 7750–7751. [Google Scholar] [CrossRef]
- Li, L.Y.; Li, G.B.; Wang, Y.X.; Liao, F.H.; Lin, J.H. Bismuth borates: One-dimensional borate chains and nonlinear optical properties. Chem. Mater. 2005, 17, 4174–4180. [Google Scholar] [CrossRef]
- Ok, K.M. Toward the rational design of novel noncentrosymmetric materials: Factors influencing the framework structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.G.; Poeppelmeier, K.R. Chemistry-inspired adaptable framework structures. Acc. Chem. Res. 2017, 50, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Lei, B.H.; Zhang, W.Y.; Pan, S.L. Module-analysis-assisted design of deep ultraviolet fluorooxoborates with extremely large gap and high structural stability. Chem. Mater. 2019, 31, 2807–2813. [Google Scholar] [CrossRef]
- Song, J.L.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. A facile synthetic route to a new SHG material with two types of parallel π-conjugated planar triangular units. Angew. Chem. Int. Ed. 2015, 54, 3679–3682. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, C.L.; Mao, F.F.; Feng, J.H.; Mao, J.G. A facile route to nonlinear optical materials: Three-site aliovalent substitution involving one cation and two anions. Angew. Chem. Int. Ed. 2019, 131, 2120–2124. [Google Scholar] [CrossRef]
- Jing, Q.; Yang, G.; Chen, Z.H.; Dong, X.Y.; Shi, Y.J. A joint strategy to evaluate the microscopic origin of the second-harmonic-generation response in nonpolar ABCO3F Compounds. Inorg. Chem. 2018, 57, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.H.; Yang, Z.H.; Yu, H.W.; Cao, C.; Li, Z.; Hu, C.; Poeppelmeier, K.R.; Pan, S.L. Module-guided design scheme for deep-ultraviolet nonlinear optical materials. J. Am. Chem. Soc. 2018, 140, 10726–10733. [Google Scholar] [CrossRef]
- Shen, Y.G.; Zhao, S.G.; Luo, J.H. The role of cations in second-order nonlinear optical materials based on π-conjugated [BO3]3− groups. Coord. Chem. Rev. 2018, 366, 1–28. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Pan, S.L.; Yang, Z.H.; Wang, Z.; Bian, Q.; Hou, X.L.; Yu, H.W.; Zhang, F.F.; Wu, K.; et al. Na3Ba2(B3O6)2F: Next generation of deep-ultraviolet birefringent materials. Cryst. Growth Des. 2015, 15, 523–529. [Google Scholar] [CrossRef]
- Huang, H.W.; Yao, J.Y.; Lin, Z.S.; Wang, X.Y.; He, R.; Yao, W.J.; Zhai, N.X.; Chen, C.T. Molecular engineering design to resolve the layering habit and polymorphism problems in deep UV NLO crystals: New structures in MM’Be2B2O6F (M = Na, M’ = Ca; M = K, M’ = Ca, Sr). Chem. Mater. 2011, 23, 5457–5463. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, G.L.; Wang, X.Y.; Xu, Z.Y. Deep-UV nonlinear optical crystal KBe2BO3F2-discovery, growth, optical properties and applications. Appl. Phys. B 2009, 97, 9–25. [Google Scholar] [CrossRef]
- Eimerl, D.; Davis, L.; Velsko, S.; Graham, E.K.; Zalkin, A. Optical, mechanical and thermal-properties of barium borate. J. Appl. Phys. 1987, 62, 1968–1983. [Google Scholar] [CrossRef]
- Cyranoski, D. Materials science: China’s crystal cache. Nature 2009, 457, 953–955. [Google Scholar] [CrossRef]
- Becker, P. A contribution to borate crystal chemistry: Rules for the occurrence of polyborate anion types. Z. Kristallogr. 2001, 216, 523–533. [Google Scholar] [CrossRef]
- Zhao, S.G.; Gong, P.F.; Bai, L.; Xu, X.; Zhang, S.Q.; Sun, Z.H.; Lin, Z.S.; Hong, M.C.; Chen, C.T.; Luo, J.H. Beryllium-free Li4Sr(BO3)2 for deep-ultraviolet nonlinear optical applications. Nat. Commun. 2014, 5, 4019. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.H.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. Na2RE2TeO4(BO3)2 (RE = Y, Dy-Lu): Luminescent and structural studies on a series of mixed metal borotellurates. Inorg. Chem. 2015, 54, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Cheng, W.D.; Zhang, H.; Geng, L.; Lin, C.S.; He, Z.Z. A strong second-harmonic generation material Cd4BiO(BO3)3 originating from 3-chromophore asymmetric structures. J. Am. Chem. Soc. 2010, 132, 1508–1509. [Google Scholar] [CrossRef]
- Hao, Y.C.; Xu, X.; Kong, F.; Song, J.L.; Mao, J.G. PbCd2B6O12 and EuZnB5O10: Syntheses, crystal structures and characterizations of two new mixed metal borates. CrystEngComm 2014, 16, 7689–7695. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, Q.; Wu, H.Q.; Zhou, L.J.; Yang, G.Y. Nonlinear optical metal borates containing two types of oxoboron clusters. Chem. Eur. J. 2013, 19, 17662–17667. [Google Scholar] [CrossRef]
- Song, H.M.; Wang, N.Z.; Jiang, X.X.; Fu, Y.; Li, Y.F.; Liu, W.; Lin, Z.S.; Yao, J.Y.; Zhang, G.C. Growth, crystal structures, and characteristics of Li5ASrMB12O24 (A = Zn, Mg; M = Al, Ga) with [MB12O24] frameworks. Inorg. Chem. 2019, 58, 1016–1019. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wei, Q.; Lin, Z.E.; Meng, Q.; He, H.; Yang, B.F.; Yang, G.Y. A 3D aluminoborate open framework interpenetrated by 2D zinc-amine coordination-polymer networks in its 11-ring channels. Angew. Chem. Int. Ed. 2014, 53, 7188–7191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, W.H.; Rong, C.; Yang, G.Y. A series of open-framework aluminoborates templated by transition-metal complexes. Chem. Eur. J. 2010, 16, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, L.J.; Jin, S.F.; Yao, W.J.; Zhang, Y.H.; Chen, C.T. Deep-ultraviolet nonlinear optical materials: Na2Be4B4O11 and LiNa5Be12B12O33. J. Am. Chem. Soc. 2013, 135, 18319–18322. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Wu, H.P.; Pan, S.L.; Yang, Z.H.; Hou, X.L.; Su, X.; Jing, Q.; Poeppelmeier, K.R.; Rondinelli, J.M. Cs3Zn6B9O21: A chemically benign member of the KBBF family exhibiting the largest second harmonic generation response. J. Am. Chem. Soc. 2014, 136, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Zhang, J.; Zhang, S.Q.; Sun, Z.H.; Lin, Z.S.; Wu, Y.C.; Hong, M.C.; Luo, J.H. A new UV nonlinear optical material CsZn2B3O7: ZnO4 tetrahedra double the efficiency of second-harmonic generation. Inorg. Chem. 2014, 53, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Mutailipu, M.; Zhang, M.; Wu, H.P.; Yang, Z.H.; Shen, Y.H.; Sun, J.L.; Pan, S.L. Ba3Mg3(BO3)3F3 polymorphs with reversible phase transition and high performances as ultraviolet nonlinear optical materials. Nat. Commun. 2018, 9, 3089. [Google Scholar] [CrossRef]
- Zhao, S.G.; Kang, L.; Shen, Y.G.; Wang, X.D.; Asghar, M.A.; Lin, Z.S.; Xu, Y.Y.; Zeng, S.Y.; Hong, M.C.; Luo, J.H. Designing a beryllium-free deep-ultraviolet nonlinear optical material without a structural instability problem. J. Am. Chem. Soc. 2016, 138, 2961–2964. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Bai, L.; Li, B.X.; Zhao, S.G.; Shen, Y.G.; Li, X.F.; Ding, Q.R.; Ji, C.M.; Lin, Z.S.; Luo, J.H. Crystal growth and optical properties of beryllium-free nonlinear optical crystal K3Ba3Li2Al4B6O20F. Cryst. Growth Des. 2018, 18, 1168–1172. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Li, B.X.; Zhao, S.G.; Liu, X.T.; Wu, Z.Y.; Shen, Y.G.; Li, X.F.; Ding, Q.R.; Ji, C.M.; Luo, J.H. Physical properties of a promising nonlinear optical crystal K3Ba3Li2Al4B6O20F. Cryst. Growth Des. 2018, 18, 7368–7372. [Google Scholar] [CrossRef]
- Wu, H.P.; Yu, H.W.; Pan, S.L.; Halasyamani, P.S. Deep-ultraviolet nonlinear-optical material K3Sr3Li2Al4B6O20F: Addressing the structural instability problem in KBe2BO3F2. Inorg. Chem. 2017, 56, 8755–8758. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Young, J.; Wu, H.P.; Zhang, W.G.; Rondinelli, J.M.; Halasyamani, P.S. The next-generation of nonlinear optical materials: Rb3Ba3Li2Al4B6O20F-synthesis, characterization, and crystal growth. Adv. Opt. Mater. 2017, 5, 1700840. [Google Scholar] [CrossRef]
- Shen, Y.G.; Zhao, S.G.; Yang, Y.; Cao, L.L.; Wang, Z.J.; Zhao, B.Q.; Sun, Z.H.; Lin, Z.S.; Luo, J.H. A new KBBF-family nonlinear optical material with strong interlayer bonding. Cryst. Growth Des. 2017, 17, 4422–4427. [Google Scholar] [CrossRef]
- Meng, X.H.; Liang, F.; Xia, M.J.; Lin, Z.S. Beryllium-free nonlinear-optical crystals A3Ba3Li2Ga4B6O20F (A = K and Rb): Members of the Sr2Be2(BO3)2O family with a strong covalent connection between the ∞2[Li2Ga4B6O20F]9− double layers. Inorg. Chem. 2018, 57, 5669–5676. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Wu, H.P.; Pan, S.L.; Zhang, B.B.; Dong, L.Y.; Han, S.J.; Yang, Z.H. Pb4Zn2B10O21: A congruently melting lead zinc borate with a novel [B10O24] anionic group and an interesting [Pb4O12]∞ chain. New J. Chem. 2014, 38, 285–291. [Google Scholar] [CrossRef]
- Mutailipu, M.; Li, Z.; Zhang, M.; Hou, D.W.; Yang, Z.H.; Zhang, B.B.; Wu, H.P.; Pan, S.L. The mechanism of large second harmonic generation enhancement activated by Zn2+ substitution. Phys. Chem. Chem. Phys. 2016, 18, 32931–32936. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; An, D.H.; Zhang, M.; Hu, C.; Mutailipu, M.; Yang, Z.H.; Lu, X.Q.; Pan, S.L. Li6Zn3(BO3)4: A new zincoborate featuring vertex-, edge- and face-sharing LiO4 tetrahedra and exhibiting reversible phase transitions. Inorg. Chem. Front. 2017, 4, 1100–1107. [Google Scholar] [CrossRef]
- Yang, G.S.; Gong, P.F.; Lin, Z.S.; Ye, N. AZn2BO3X2 (A = K, Rb, NH4; X = Cl, Br): New members of KBBF family exhibiting large SHG response and the enhancement of layer interaction by modified structures. Chem. Mater. 2016, 28, 9122–9131. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.J.; Wang, X.Y.; Li, R.K.; Chen, C.T. Beryllium-free KBBF family of nonlinear-optical crystals: AZn2BO3X2 (A = Na, K, Rb; X = Cl, Br). Inorg. Chem. 2016, 55, 12496–12499. [Google Scholar] [CrossRef]
- Yu, H.W.; Zhang, W.G.; Young, J.; Rondinelli, J.M.; Halasyamani, P.S. Design and synthesis of the beryllium-free deep-ultraviolet nonlinear optical material Ba3(ZnB5O10)PO4. Adv. Mater. 2015, 27, 7380–7385. [Google Scholar] [CrossRef]
- Li, R.K.; Chen, P. Cation coordination control of anionic group alignment to maximize SHG effects in the BaMBO3F (M = Zn, Mg) series. Inorg.Chem. 2010, 49, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.H.; Xia, M.J.; Li, R.K. Ba5Zn4(BO3)6: A nonlinear-optical material with reinforced interlayer connections and large second-harmonic-generation response. Inorg. Chem. 2017, 56, 11458–11461. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W.; Koliha, L.J. A new noncentrosymmetric orthoborate [Ba2Zn(BO3)2]. Mater. Res. Bull. 1994, 29, 1203–1210. [Google Scholar] [CrossRef]
- Zhang, W.G.; Yu, H.W.; Wu, H.P.; Halasyamani, P.S. Crystal growth and associated properties of a nonlinear optical crystal-Ba2Zn(BO3)2. Crystals 2016, 6, 68. [Google Scholar] [CrossRef]
- Lou, Y.F.; Li, D.D.; Li, Z.L.; Zhang, H.; Jin, S.F.; Chen, X.L. Unidirectional thermal expansion in KZnB3O6: Role of alkali metals. Dalton Trans. 2015, 44, 19763–19767. [Google Scholar] [CrossRef]
- Lou, Y.F.; Li, D.D.; Li, Z.L.; Jin, S.F.; Chen, X.L. Unidirectional thermal expansion in edge-sharing BO4 tetrahedra contained KZnB3O6. Sci. Rep. 2015, 5, 10996. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Molokeev, M.S.; Gong, P.F.; Yang, Y.; Wang, W.; Wang, S.H.; Wu, S.F.; Wang, Y.X.; Huang, R.J.; Li, L.F.; et al. Near-zero thermal expansion and high ultraviolet transparency in a borate crystal of Zn4B6O13. Adv. Mater. 2016, 28, 7936–7940. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, X.X.; Gong, P.F.; Molokeev, M.S.; Li, X.D.; Li, Y.C.; Wu, X.; Wu, Y.C.; Lin, Z.S. High mechanical strength in Zn4B6O13 with an unique sodalite-cage structure. RSC Adv. 2017, 7, 2038–2043. [Google Scholar] [CrossRef]
- Jin, S.F.; Cai, G.M.; Wang, W.Y.; He, M.; Wang, S.C.; Chen, X.L. Stable oxoborate with edge-sharing BO4 tetrahedra synthesized under ambient pressure. Angew. Chem. Int. Ed. 2010, 49, 4967–4970. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, J.Y.; Zhang, J.X.; Fu, P.Z.; Wu, Y.C. Potassium zinc borate, KZnB3O6. Acta Crystallogr. 2010, 66, i45. [Google Scholar] [CrossRef]
- Sohr, G.; Perfler, L.; Huppertz, H. The high-pressure thallium triborate HP-TlB3O5. Z. Naturforsch. 2014, 69, 1260–1268. [Google Scholar] [CrossRef]
- Neumair, S.C.; Vanicek, S.; Kaindl, R.; Többens, D.M.; Martineau, C.; Taulelle, F.; Senker, J.; Huppertz, H. HP-KB3O5 highlights the structural diversity of borates: Corner-sharing BO3/BO4 groups in combination with edge-sharing BO4 tetrahedra. Eur. J. Inorg. Chem. 2011, 2011, 4147–4152. [Google Scholar] [CrossRef]
- Sohr, G.; Neumair, S.C.; Huppertz, H. High-pressure synthesis and characterization of the alkali metal borate HP-RbB3O5. Z. Naturforsch. 2012, 67, 1197–1204. [Google Scholar] [CrossRef]
- Sohr, G.; Többens, D.M.; Schmedt auf der Günne, J.; Huppertz, H. HP-CsB5O8: Synthesis and characterization of an outstanding borate exhibiting the simultaneous linkage of all structural units of borates. Chem. Eur. J. 2014, 20, 17059–17067. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.A.; Chen, Y.J.; Sun, C.; Chang, X.A.; Xiao, W.Q. Synthesis, crystal structure, spectrum properties, and electronic structure of a new three-borate Ba4Na2Zn4(B3O6)2(B12O24) with two isolated types of blocks: 3[3Δ] and 3[2Δ + 1T]. J. Alloys Compd. 2013, 568, 60–67. [Google Scholar] [CrossRef]
- Pauling, L. The principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Burdett, J.K.; McLarnan, T.J. An orbital interpretation of Pauling’s rules. Am. Mineral. 1984, 69, 601–621. [Google Scholar]
- Mutailipu, M.; Su, X.; Zhang, M.; Yang, Z.H.; Pan, S.L. Ban+2Znn(BO3)n(B2O5)Fn (n = 1, 2): New members of the zincoborate fluoride series with two kinds of isolated B-O units. Inorg. Chem. Front. 2017, 4, 281–288. [Google Scholar] [CrossRef]
- Busche, S.; Bluhm, K. Zur synthese und kristallstruktur von dibariumkaliumtrizinkborat Ba2KZn3(B3O6)(B6O13)/synthesis and crystal structure of di-barium potassium tri-zinc borate Ba2KZn3(B3O6)(B6O13). Z. Naturforsch. 1996, 51, 319–324. [Google Scholar] [CrossRef]
- Chen, X.A.; Chen, Y.J.; Wu, L.; Chang, X.A.; Xiao, W.Q. Synthesis, crystal structure, and spectrum properties of a new borate Ba4K2Zn5(B3O6)3(B9O19) with two isolated types of blocks: 3[3Δ] and 3[2Δ + 1T] + 3Δ + 3[2Δ + 1T]. Solid State Sci. 2014, 27, 47–54. [Google Scholar] [CrossRef]
- Barbier, J.; Penin, N.; Cranswick, L.M. Melilite-type borates Bi2ZnB2O7 and CaBiGaB2O7. Chem. Mater. 2005, 17, 3130–3136. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.A.; Chang, X.A.; Zang, H.G.; Xiao, W.Q. Synthesis, Crystal structure and optical properties of non-centrosymmetric borate, Bi2ZnB2O7. J. Syn. Cryst. 2007, 36, 1005–1010. [Google Scholar]
- Li, F.; Hou, X.L.; Pan, S.L.; Wang, X. Growth, structure, and optical properties of a congruent melting oxyborate, Bi2ZnOB2O6. Chem. Mater. 2009, 21, 2846–2850. [Google Scholar] [CrossRef]
- Yu, H.W.; Wu, H.P.; Jing, Q.; Yang, Z.H.; Halasyamani, P.S.; Pan, S.L. Polar Polymorphism: α-, β-, and γ-Pb2Ba4Zn4B14O31-Synthesis, characterization, and nonlinear optical properties. Chem. Mater. 2015, 27, 4779–4788. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhang, M.; Hu, C.; Yang, Z.H.; Pan, S.L. Ba2ZnSc(BO3)3 and Ba4Zn5Sc2(BO3)8: First examples of borates in the Zn-Sc-B-O system featuring special structure configurations. Inorg. Chem. Front. 2018, 5, 1787–1794. [Google Scholar] [CrossRef]
- Huang, Z.J.; Pan, S.L.; Yang, Z.H.; Yu, H.W.; Dong, X.Y.; Zhao, W.W.; Dong, L.Y.; Su, X. Pb8M(BO3)6 (M = Zn, Cd): Two new isostructural lead borates compounds with two-dimensional ∞[Pb8B6O18]2− layer structure. Solid State Sci. 2013, 15, 73–78. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Zhou, Y.; Chen, Y.; Li, C.; Zhu, Y.; Xu, Z.; Chen, C. 12.95 mW sixth harmonic generation with KBe2BO3F2 crystal. Appl. Phys. B 2008, 91, 95–97. [Google Scholar] [CrossRef]
- Wu, B.C.; Tang, D.Y.; Ye, N.; Chen, C.T. Linear and nonlinear optical properties of the KBe2BO3F2 (KBBF) crystal. Opt. Mater. 1996, 5, 105–109. [Google Scholar] [CrossRef]
- Chen, C.T. Recent advances in deep and vacuum-UV harmonic generation with KBBF crystal. Opt. Mater. 2004, 26, 425–429. [Google Scholar] [CrossRef]
- Jiang, X.X.; Luo, S.Y.; Kang, L.; Gong, P.F.; Huang, H.W.; Wang, S.C.; Lin, Z.S.; Chen, C.T. First-principles evaluation of the alkali and/or alkaline earth beryllium borates in deep ultraviolet nonlinear optical applications. ACS Photonics 2015, 2, 1183–1191. [Google Scholar] [CrossRef]
- Zou, G.H.; Lin, C.S.; Jo, H.; Nam, G.; You, T.S.; Ok, K.M. Pb2BO3Cl: A tailor-made polar lead borate chloride with very strong second harmonic generation. Angew. Chem. Int. Ed. 2016, 55, 12078–12082. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Koocher, N.Z.; Rondinelli, J.M.; Halasyamani, P.S. Pb2BO3I: A borate iodide with the largest second-harmonic generation (SHG) response in the KBe2BO3F2 (KBBF) family of nonlinear optical (NLO) materials. Angew. Chem. Int. Ed. 2018, 57, 6100–6103. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Song, Y.X.; Liang, F.; Ye, N.; Lin, Z.S. Pb2BO3Br: A novel nonlinear optical lead borate bromine with a KBBF-type structure exhibiting strong nonlinear optical response. Inorg. Chem. Front. 2018, 5, 916–921. [Google Scholar] [CrossRef]
- Tran, T.T.; Koocher, N.Z.; Rondinelli, J.M.; Halasyamani, P.S. Beryllium-free β-Rb2Al2B2O7 as a possible deep-ultraviolet nonlinear optical material replacement for KBe2BO3F2. Angew. Chem. Int. Ed. 2017, 56, 2969–2973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Gong, P.F.; Luo, S.Y.; Liu, S.J.; Li, L.N.; Asghar, M.A.; Khan, T.; Hong, M.C.; Lin, Z.S.; Luo, J.H. Beryllium-free Rb3Al3B3O10F with reinforced interlayer bonding as a deep-ultraviolet nonlinear optical crystal. J. Am. Chem. Soc. 2015, 137, 2207–2210. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jiang, X.X.; Duan, M.H.; Hou, Z.Y.; Tang, C.C.; Xia, M.J.; Liu, L.J.; Lin, Z.S.; Fan, F.D.; Bai, L.; et al. Deep-ultraviolet nonlinear optical crystal Cs2Al2(B3O6)2O: A benign member of the Sr2Be2(BO3)2O family with [Al2(B3O6)2O]2− double layers. Chem. Eur. J. 2018, 24, 7856–7860. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Zhang, M.; Pan, S.L. BaLiZn3(BO3)3: A new member of the KBe2BO3F2 family possessing dense BO3 triangles and the smallest interlayer distance. New J. Chem. 2018, 42, 12365–12368. [Google Scholar] [CrossRef]
- Duan, M.H.; Xia, M.J.; Li, R.K. BaLiZn3B3O9: A mixed-cation KBe2BO3F2-type zinc-borate with a (LiZn3B3O9)∞ network. Eur. J. Inorg. Chem. 2018, 32, 3686–3689. [Google Scholar] [CrossRef]
- Jiao, Z.W.; Zhang, F.; Yan, Q.F.; Shen, D.Z.; Shen, G.Q. Synthesis, structure characterization and fluorescence property of a new fluoride borate crystal, CdZn2KB2O6F. J. Solid State Chem. 2009, 182, 3063–3066. [Google Scholar] [CrossRef]
- Zhang, F.; Jiao, Z.W.; Shen, D.Z.; Shen, G.Q.; Wang, X.Q. CdZn2KB2O6F, a new fluoride borate crystal. Acta Crystallogr. 2010, 66, i1–i3. [Google Scholar] [CrossRef]
- Wang, S.C.; Ye, N. Na2CsBe6B5O15: An alkaline beryllium borate as a deep-UV nonlinear optical crystal. J. Am. Chem. Soc. 2011, 133, 11458–11461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Ye, N.; Li, W.; Zhao, D. Alkaline beryllium borate NaBeB3O6 and ABe2B3O7 (A = K, Rb) as UV nonlinear optical crystals. J. Am. Chem. Soc. 2010, 132, 8779–8786. [Google Scholar] [CrossRef] [PubMed]
- Knyrim, J.S.; Becker, P.; Johrendt, D.; Huppertz, H. A new non-centrosymmetric modification of BiB3O6. Angew. Chem. Int. Ed. 2006, 45, 8239–8241. [Google Scholar] [CrossRef] [PubMed]
- An, D.H.; Kong, Q.R.; Zhang, M.; Yang, Y.; Li, D.N.; Yang, Z.H.; Pan, S.L.; Chen, H.M.; Su, Z.; Sun, Y.; et al. Versatile coordination mode of LiNaB8O13 and α- and β-LiKB8O13 via the flexible assembly of four-connected B5O10 and B3O7 Groups. Inorg. Chem. 2016, 55, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Yao, J.Y.; Lin, Z.S.; Wang, X.Y.; He, R.; Yao, W.J.; Zhai, N.X.; Chen, C.T. NaSr3Be3B3O9F4: A promising deep-ultraviolet nonlinear optical material resulting from the cooperative alignment of the [Be3B3O12F]10− anionic group. Angew. Chem. Int. Ed. 2011, 50, 9141–9144. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, H.; von der Eltz, B. Multianvil high-pressure synthesis of Dy4B6O15: The first oxoborate with edge-sharing BO4 tetrahedra. J. Am. Chem. Soc. 2002, 124, 9376–9377. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, H. High-pressure preparation, crystal structure, and properties of RE4B6O15 (RE = Dy, Ho) with an extension of the “fundamental building block”-descriptors. Z. Naturforsch. 2003, 58, 278–290. [Google Scholar] [CrossRef]
- Emme, H.; Huppertz, H. High-pressure preparation, crystal structure, and properties of α-(RE)2B4O9 (RE = Eu, Gd, Tb, Dy): Oxoborates displaying a new type of structure with edge-sharing BO4 tetrahedra. Chem. Eur. J. 2003, 9, 3623–3633. [Google Scholar] [CrossRef]
- Emme, H.; Huppertz, H. High-pressure syntheses of α-RE2B4O9 (RE = Sm, Ho), with a structure type displaying edge-sharing BO4 tetrahedra. Acta Cryst. 2005, C61, i29–i31. [Google Scholar]
- Knyrim, J.S.; Roeßner, F.; Jakob, S.; Johrendt, D.; Kinski, I.; Glaum, R.; Huppertz, H. Formation of edge-sharing BO4 tetrahedra in the high-pressure borate HP-NiB2O4. Angew. Chem. Int. Ed. 2007, 46, 9097–9100. [Google Scholar] [CrossRef]
- Neumair, S.C.; Kaindl, R.; Huppertz, H. Synthesis and crystal structure of the high-pressure cobalt borate HP-CoB2O4. Z. Naturforsch. 2010, 65, 1311–1317. [Google Scholar] [CrossRef]
- Jen, I.H.; Lee, Y.C.; Tsai, C.E.; Lii, K.H. Edge-sharing BO4 tetrahedra in the structure of hydrothermally synthesized barium borate: α-Ba3[B10O17(OH)2]. Inorg. Chem. 2019, 58, 4085–4088. [Google Scholar] [CrossRef] [PubMed]
- Mutailipu, M.; Zhang, M.; Li, H.; Fan, X.; Yang, Z.H.; Jin, S.F.; Wang, G.; Pan, S.L. Li4Na2CsB7O14: A new edge-sharing [BO4]5− tetrahedra containing borate with high anisotropic thermal expansion. Chem. Commun. 2019, 55, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.J.; Han, J.; Cheng, S.C.; Yu, S.J.; Yang, Z.H.; Pan, S.L. Transformation of the B-O units from corner-sharing to edge-sharing linkages in BaMBO4 (M = Ga, Al). Inorg. Chem. 2019, 58, 8237–8244. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Huang, C.M.; Tudi, A.; Hu, S.S.; Yang, Z.H.; Pan, S.L. β-CsB9O14: A triple-layered borate with edge-sharing BO4 tetrahedra exhibiting a short cutoff edge and a large birefringence. Chem. Eur. J. 2019. [Google Scholar] [CrossRef]
- Yang, L.; Fan, W.L.; Li, Y.L.; Sun, H.G.; Wei, L.; Cheng, X.F.; Zhao, X. Theoretical insight into the structural stability of KZnB3O6 polymorphs with different BOx polyhedral networks. Inorg. Chem. 2012, 51, 6762–6770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. New second-order NLO materials based on polymeric borate clusters and GeO4 tetrahedra: A combined experimental and theoretical study. Inorg. Chem. 2011, 50, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, J.J.; He, C.; Cheng, J.W.; Yang, G.Y. Deep-ultraviolet nonlinear optics in a borate framework with 21-ring channels. Chem. Eur. J. 2016, 22, 10759–10762. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Mori, Y.; Yoshimura, M.; Yap, Y.K.; Kamimura, T. Recent development of nonlinear optical borate crystals: Key materials for generation of visible and UV light. Mater. Sci. Eng. R 2000, 30, 1–54. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, S.L. Recent development of metal borate halides: Crystal chemistry and application in second-order NLO materials. Coord. Chem. Rev. 2016, 323, 15–35. [Google Scholar] [CrossRef]
- Wu, C.; Yang, G.; Humphrey, M.G.; Zhang, C. Recent advances in ultraviolet and deep-ultraviolet second-order nonlinear optical crystals. Coord. Chem. Rev. 2018, 375, 459–488. [Google Scholar] [CrossRef]
- Dotsenko, V.P.; Berezovskaya, I.V.; Efryushina, N.P.; Voloshinovskii, A.S.; Stryganyuk, G.B. Position of the optical absorption edge of alkaline earth borates. Opt. Mater. 2009, 31, 1428–1433. [Google Scholar] [CrossRef]
- Huang, J.H.; Jin, C.C.; Xu, P.L.; Gong, P.F.; Lin, Z.S.; Cheng, J.W.; Yang, G.Y. Li2CsB7O10(OH)4: A deep-ultraviolet nonlinear-optical mixed-alkaline borate constructed by unusual heptaborate anions. Inorg. Chem. 2019, 58, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.H.; Ma, Z.J.; Wu, K.C.; Ye, N. Cadmium-rare earth oxyborates Cd4ReO(BO3)3 (Re = Y, Gd, Lu): Congruently melting compounds with large SHG responses. J. Mater. Chem. 2012, 22, 19911–19918. [Google Scholar] [CrossRef]
- Zhao, S.G.; Zhang, G.C.; Yao, J.Y.; Wu, Y.C. K3YB6O12: A new nonlinear optical crystal with a short UV cutoff edge. Mater. Res. Bull. 2012, 47, 3810–3813. [Google Scholar] [CrossRef]
- Feng, J.H.; Xu, X.; Hu, C.L.; Mao, J.G. K6ACaSc2(B5O10)3 (A = Li, Na, Li0.7Na0.3): Nonlinear-optical materials with short UV cutoff edges. Inorg. Chem. 2019, 58, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Mutailipu, M.; Xie, Z.Q.; Su, X.; Zhang, M.; Wang, Y.; Yang, Z.H.; Janjua, M.R.S.A.; Pan, S.L. Chemical cosubstitution-oriented design of rare-earth borates as potential ultraviolet nonlinear optical materials. J. Am. Chem. Soc. 2017, 139, 18397–18405. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Mutailipu, M.; He, G.J.; Han, G.P.; Wang, Y.; Yang, Z.H.; Zhang, M.; Pan, S.L. A series of rare-earth borates K7MRE2B15O30 (M = Zn, Cd, Pb; RE = Sc, Y, Gd, Lu) with large second harmonic generation responses. Chem. Mater. 2018, 30, 2414–2423. [Google Scholar] [CrossRef]
- Wu, H.P.; Pan, S.L.; Yu, H.W.; Chen, Z.H.; Zhang, F.F. Synthesis, crystal structure and properties of a new congruently melting compound, K3ZnB5O10. Solid State Sci. 2012, 14, 936–940. [Google Scholar] [CrossRef]
- Baiheti, T.; Han, S.J.; Tudi, A.; Yang, Z.H.; Yu, H.H.; Pan, S.L. From centrosymmetric to noncentrosymmetric: Cation-directed structural evolution in X3ZnB5O10 (X = Na, K, Rb) and Cs12Zn4(B5O10)4 crystals. Inorg. Chem. Front. 2019, 6, 1461–1467. [Google Scholar] [CrossRef]
- Halasyamani, P.S.; Zhang, W.G. Viewpoint: Inorganic materials for UV and deep-UV nonlinear-optical applications. Inorg. Chem. 2017, 56, 12077–12085. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Yu, H.W.; Yang, Z.H.; Hou, X.L.; Su, X.; Pan, S.L.; Poeppelmeier, K.R.; Rondinelli, J.M. Designing a deep-ultraviolet nonlinear optical material with a large second harmonic generation response. J. Am. Chem. Soc. 2013, 135, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kuroda, I.; Nakajima, S.; Sasaki, T.; Nakai, S. New nonlinear optical crystal: Cesium lithium borate. Appl. Phys. Lett. 1995, 67, 1818–1820. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Mori, Y.; Kuroda, I.; Nakajima, S.; Yamaguchi, K.; Watanabe, S.; Nakai, S. Caesium lithium borate: A new nonlinear optical crystal. Acta Crystallogr. C 1995, 51, 2222–2224. [Google Scholar] [CrossRef]
- Xia, M.J.; Li, R.K. Growth, structure and optical properties of nonlinear optical crystal BaZnBO3F. J. Solid State Chem. 2016, 233, 58–61. [Google Scholar] [CrossRef]
- Wang, G.F.; Wu, Y.C.; Fu, P.Z.; Liang, X.Y.; Xu, Z.Y.; Chen, C.T. Crystal growth and properties of β-Zn3BPO7. Chem. Mater. 2002, 14, 2044–2047. [Google Scholar] [CrossRef]
- Dagdale, S.R.; Muley, G.G. Synthesis and characterization of a novel nonlinear optical material Mg2Na2ZnB4O10. Proce. Technol. 2016, 24, 682–688. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, Y.C.; Li, R.K. The anionic group theory of the non-linear optical effect and its applications in the development of new high-quality NLO crystals in the borate series. Int. Rev. Phys. Chem. 1989, 8, 65–91. [Google Scholar] [CrossRef]
- Ye, N.; Chen, Q.X.; Wu, B.C.; Chen, C.T. Searching for new nonlinear optical materials on the basis of the anionic group theory. J. Appl. Phys. 1998, 84, 555–558. [Google Scholar] [CrossRef]
- Li, F.; Pan, S.L.; Hou, X.L.; Yao, J. A novel nonlinear optical crystal Bi2ZnOB2O6. Cryst. Growth Des. 2009, 9, 4091–4095. [Google Scholar] [CrossRef]
- Sleight, A. Zero-expansion plan. Nature 2003, 425, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L.; Deng, J.X.; Xing, X.R. Negative thermal expansion in functional materials: Controllable thermal expansion by chemical modifications. Chem. Soc. Rev. 2015, 44, 3522–3567. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, R.S.; Filatov, S.K. Strong anisotropic thermal expansion in borates. Phys. Stat. Sol. B 2008, 245, 2469–2476. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Stepanov, N.K.; Levin, A.A.; Filatov, S.K.; Paufler, P.; Meyer, D.C. Crystal structure and thermal behaviour of boropollucite CsBSi2O6. Solid State Sci. 2004, 6, 629–637. [Google Scholar] [CrossRef]
- Lin, W.; Dai, G.Q.; Huang, Q.Z.; Zhen, A.; Liang, J.K. Anisotropic thermal expansion of LiB3O5. J. Phys. D: Appl. Phys. 1990, 23, 1073–1075. [Google Scholar]
- Becker, P.; Bohaty, L. Thermal expansion of bismuth triborate. Cryst. Res. Technol. 2001, 36, 1175–1180. [Google Scholar] [CrossRef]
- Yao, W.J.; Jiang, X.X.; Huang, R.J.; Li, W.; Huang, C.J.; Lin, Z.S.; Li, L.F.; Chen, C.T. Area negative thermal expansion in a beryllium borate LiBeBO3 with edge sharing tetrahedra. Chem. Commun. 2014, 50, 13499–13501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Luo, S.Y.; Kang, L.; Gong, P.F.; Yao, W.J.; Huang, H.W.; Li, W.; Huang, R.J.; Wang, W.; Li, Y.C.; et al. Isotropic negative area compressibility over large pressure range in potassium beryllium fluoroborate and its potential applications in deep ultraviolet region. Adv. Mater. 2015, 27, 4851–4857. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.Q.; Wang, Y.; Zhang, F.F.; Zhang, B.B.; Yang, Z.H.; Hou, X.L.; Pan, S.L.; Poeppelmeier, K.R. Finding the next deep-ultraviolet nonlinear optical material: NH4B4O6F. J. Am. Chem. Soc. 2017, 139, 10645–10648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Shi, G.Q.; Yang, Z.H.; Zhang, F.F.; Pan, S.L. Fluorooxoborates: Beryllium-free deep-ultraviolet nonlinear optical materials without layered growth. Angew. Chem. Int. Ed. 2017, 56, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Mutailipu, M.; Zhang, M.; Zhang, B.B.; Wang, L.Y.; Yang, Z.H.; Zhou, X.; Pan, S.L. SrB5O7F3: Functionalized with [B5O9F3]6− chromophores: Accelerating the rational design of deep-ultraviolet nonlinear optical materials. Angew. Chem. Int. Ed. 2018, 57, 6095–6099. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Pan, S.L.; Hou, X.L.; Zhou, Z.X. Growth of Bi2ZnOB2O6 crystal by the Czochralski method. J. Cryst. Growth 2010, 312, 2383–2385. [Google Scholar] [CrossRef]
- Yu, H.W.; Cantwell, J.; Wu, H.P.; Zhang, W.G.; Poeppelmeier, K.R.; Halasyamani, P.S. Top-seeded solution crystal growth, morphology, optical and thermal properties of Ba3(ZnB5O10)PO4. Cryst. Growth Des. 2016, 16, 3976–3982. [Google Scholar] [CrossRef]
- Liebertz, J.; Stähr, S. Zur Existenz und Einkristallzüchtung von Zn3BPO7 und Mg3BPO7. Z. Kristallogr. 1982, 160, 135–137. [Google Scholar] [CrossRef]
- Wang, G.F.; Fu, P.Z.; Wu, Y.C.; Chen, C.T. Study on pulling growth of β-Zn3BPO7 crystal. J. Synth. Cryst. 2000, 29, 130–133. [Google Scholar]
- Wu, Y.C.; Wang, G.F.; Fu, P.Z.; Liang, X.Y.; Xu, Z.Y.; Chen, C.T. A new nonlinear optical crystal β-Zn3BPO7. J. Cryst. Growth 2001, 229, 205–207. [Google Scholar] [CrossRef]





















| Compounds | Space Group | Structural Features | Second Harmonic Generation (SHG) Intensity (@ 1064nm) | Absorption Edge | Refs. |
|---|---|---|---|---|---|
| Cs3Zn6B9O21 | Cmc21 | 2∞[Zn2BO3O2] layer | 3.3 × KH2PO4 (KDP) | ~200 nm | [45] |
| KZn2BO3Cl2 | R32 | Isolated [BO3]3− (coplanar) | 3.01 × KDP | ~194 nm | [58] |
| RbZn2BO3Cl2 | R32 | Isolated [BO3]3− (coplanar) | 2.85 × KDP | ~198 nm | [58] |
| NH4Zn2BO3Cl2 | R32 | Isolated [BO3]3− (coplanar) | 2.82 × KDP | ~190 nm | [58] |
| KZn2BO3Br2 | R32 | Isolated [BO3]3− (coplanar) | 2.68 × KDP | ~209 nm | [58] |
| RbZn2BO3Br2 | R32 | Isolated [BO3]3− (coplanar) | 2.53 × KDP | <214 nm | [58] |
| Ba3(ZnB5O10)PO4 | Pmn21 | / | 4 × KDP (@ 532nm) | ~180 nm | [60] |
| Ba5Zn4(BO3)6 | Pc | 2∞[Zn4(BO3)4O6] layer | 2.6 × KDP | ~223 nm | [62] |
| Ba2Zn(BO3)2 | Pca21 | Isolated [BO3]3− | 1.5 × KDP | ~230 nm | [64] |
| Bi2ZnOB2O6 | Pba2 | Isolated [B2O5]4− + [B2O7]8− | 3–4 × KDP | ~330 nm | [83] |
| α−Pb2Ba4Zn4B14O31 | P1 | Isolated [B2O5]4− + [B6O13]8− | 0.6 × KDP | <289 nm | [84] |
| β−Pb2Ba4Zn4B14O31 | Cc | Isolated [B2O5]4− + [B6O13]8− | 1.1 × KDP | <304 nm | [84] |
| Cs12Zn4(B5O10)4 | 2∞[Zn(B5O10)] layer | 0.5 × KDP | <185 nm | [130] | |
| BaZnBO3F | P | Isolated [BO3]3− (coplanar) | 2.8 × KDP | ~223 nm | [135] |
| β-Zn3BPO7 | P | / | 1.8 × KDP | ~250 nm | [136] |
| Mg2Na2ZnB4O10 | / | / | 2.78 × KDP | ~210 nm | [137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, M.; Mutailipu, M.; Poeppelmeier, K.R.; Pan, S. Research and Development of Zincoborates: Crystal Growth, Structural Chemistry and Physicochemical Properties. Molecules 2019, 24, 2763. https://doi.org/10.3390/molecules24152763
Chen Y, Zhang M, Mutailipu M, Poeppelmeier KR, Pan S. Research and Development of Zincoborates: Crystal Growth, Structural Chemistry and Physicochemical Properties. Molecules. 2019; 24(15):2763. https://doi.org/10.3390/molecules24152763
Chicago/Turabian StyleChen, Yanna, Min Zhang, Miriding Mutailipu, Kenneth R. Poeppelmeier, and Shilie Pan. 2019. "Research and Development of Zincoborates: Crystal Growth, Structural Chemistry and Physicochemical Properties" Molecules 24, no. 15: 2763. https://doi.org/10.3390/molecules24152763
APA StyleChen, Y., Zhang, M., Mutailipu, M., Poeppelmeier, K. R., & Pan, S. (2019). Research and Development of Zincoborates: Crystal Growth, Structural Chemistry and Physicochemical Properties. Molecules, 24(15), 2763. https://doi.org/10.3390/molecules24152763