Hydrated Zinc Borates and Their Industrial Use
Abstract
:1. Introduction
2. Industrial Zinc Borates
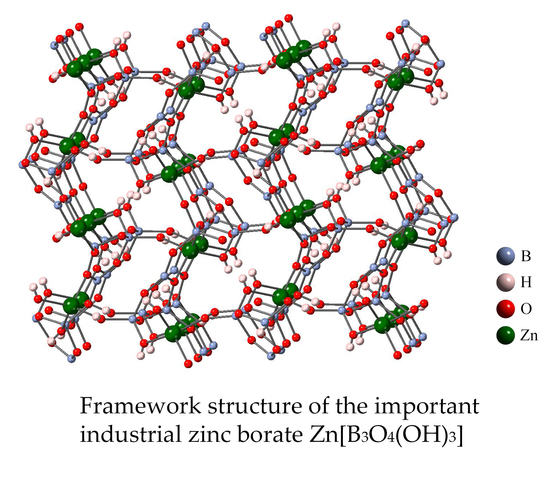
2.1. 2ZnO∙3B2O3∙3H2O (Q = 1.5) or Zn[B3O4(OH)3]
2.2. 4ZnO∙B2O3∙H2O (Q = 0.25) or Zn2(BO3)OH
2.3. 3ZnO∙3B2O3∙5H2O (Q = 1.00)
2.4. 2ZnO∙3B2O3∙7H2O (Q = 1.5) or Zn[B3O3(OH)5]∙H2O
2.5. 3ZnO∙5B2O3∙14H2O (Q = 1.67)
3. Other Hydrated Zinc Borates
3.1. Overview of Other Hydrated Zinc Borates
3.2. 16ZnO∙3B2O3∙3H2O (Q = 0.19) or Zn8(BO3)3O2(OH)3
3.3. 12ZnO∙3B2O3∙H2O (Q = 0.25) or H[Zn6O2(BO3)3] or Zn6O(OH)(BO3)3
3.4. 6ZnO∙5B2O3∙3H2O (Q = 0.83)
3.5. ZnO∙B2O3∙∼1.12H2O (Q = 1.00) or Zn(H2O)[B2O4]∙∼0.12H2O
3.6. 2ZnO∙3B2O3∙7.5H2O (Q = 1.5)
3.7. 2ZnO∙3B2O3∙4H2O (Q = 1.5) or Zn(H2O)4[B6O10]
3.8. ZnO∙5B2O3∙4.5H2O (Q = 5)
3.9. ZnO∙6B2O3∙5H2O (Q = 6) or ZnB12O14(OH)10
4. Applications
4.1. Overview of Applications
4.2. Polymer Additives
4.3. Preservative for Bio-Composites
4.4. Coatings
4.5. Ceramics and Ceramic Glazes
4.6. Agriculture
5. Conclusions
Conflicts of Interest
References
- Schubert, D.M. Boron oxides, boric acid, and borates. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Smith-Verdier, P.; Garcia-Blanco, S.; Rivoir, L. Redetermination of the structure of anhydrous zinc metaborate Zn4O(BO2)6. Z. Kristallogr. 1980, 151, 175–177. [Google Scholar] [CrossRef]
- Baur, W.H.; Tillmanns, E. The space group and crystal structure of trizinc diorthoborate. Z. Kristallogr. 1970, 181, 213–231. [Google Scholar] [CrossRef]
- Martinez-Ripoll, M.; Martinez-Carrera, S.; Garcia-Blanco, S. The crystal structure of zinc diborate ZnB4O7. Acta Cryst. 1970, 27, 672–677. [Google Scholar] [CrossRef]
- Beckett, M.A. Recent advances in crystalline hydrated borates with non-metal or transition-metal complex cations. Coord. Chem. Rev. 2016, 323, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhao, Y.; Chang, X.; Zuo, J.; Zang, H.; Xiao, W. Synthesis and crystal structures of two new hydrated borates, Zn8[(BO3)3O2(OH)3] and Pb[B5O8(OH)]∙1.5H2O. J. Solid State Chem. 2006, 179, 3911–3918. [Google Scholar] [CrossRef]
- Delahaye, T.; Boucher, F.; Paris, M.; Joubert, M.C.; Piffard, Y. Some experimental evidence that Zn4O(BO3)2 is Zn6O(OH)(BO3)3. Angew. Chem. 2006, 118, 4164–4166. [Google Scholar] [CrossRef]
- Massa, W.; Yakubovich, O.V.; Dimitrova, O.V. Proton-stabilized three-dimensional anionic framework in H[Zn6O2(BO3)3]. Acta Crystallogr. C 2006, 62, i106–i108. [Google Scholar] [CrossRef]
- Schubert, D.M. Process of Making Zinc Borate and Fire-Retarding Compositions Thereof. U.S. Patent 5,342,553, 30 August 1994. [Google Scholar]
- Schubert, D.M. Zinc Borate. U.S. Patent 5,472,644, 5 December 1995. [Google Scholar]
- Yu, Z.-T.; Xu, J.-J.; Jiang, Y.-S.; Shi, Z.; Guo, Y.; Wang, D.; Chen, J.-S. Photoluminescent and photovoltaic properties of observed in a zinc borate Zn2(OH)BO3. J. Mater. Chem. 2003, 13, 2227–2233. [Google Scholar] [CrossRef]
- Lehmann, H.A.; Sperschneider, K.; Kessler, G. Uber wasserhaltige zinkborate. Z. Anorg. Chem. 1967, 354, 37–43. [Google Scholar] [CrossRef]
- Choudhury, A.; Neeraj, S.; Natarajan, S.; Rao, C.N.R. An open framework zincborate formed by Zn6O12O24 clusters. J. Chem. Soc. Dalton Trans. 2002, 1535–1538. [Google Scholar] [CrossRef]
- Nies, N.P. Zinc Borate of Low Hydration and Method of Producing Same. U.S. Patent 3,549,316, 22 December 1970. [Google Scholar]
- Schubert, D.M.; Alam, F.; Visi, M.Z.; Knobler, C.B. Structural characterization and chemistry of the industrially important zinc borate, Zn[B3O4(OH)3]. Chem. Mater. 2003, 15, 866–871. [Google Scholar] [CrossRef]
- Ozols, J.; Tetere, I.; Ievins, A. Structure of zinc triborate. Latv. PSR Zinat. Akad. Vestis Kim. Ser. 1969, 2, 244–245. [Google Scholar]
- Putnins, J.; Ievins, A. Trizinc decaborate. Latv. Valsts Univ. Kim. Fak. Zinatniskie Raksti 1958, 22, 69–71. [Google Scholar]
- Pan, R.; Yang, B.-F.; Wang, G.-M.; Yang, G.-Y. [Zn(H2O)4][B6O10]: An open-framework borate with acentric structure. Inorg. Chem. Commun. 2017, 76, 15–17. [Google Scholar] [CrossRef]
- Yang, D.; Cong, R.; Gao, W.; Yang, T. Boric acid flux synthesis, structure and magnetic property of MB12O14(OH)10 (M = Mn, Fe, Zn). J. Solid State Chem. 2013, 201, 29–34. [Google Scholar] [CrossRef]
- Eltepe, H.E. The Development of Zinc Borate Production. Master’s Thesis, İzmir Institute of Technology, İzmir, Turkey, 2004. [Google Scholar]
- Eltepe, H.E.; Balköse, D.; Ülkü, S. Effect of temperature and time on zinc borate species formed from zinc oxide and boric acid in aqueous medium. Ind. Eng. Chem. Res. 2007, 46, 2367–2371. [Google Scholar] [CrossRef]
- Gürhan, D.; Çakal, G.Ö.; Eroglu, İ.; Özkar, S. Improved synthesis of fine zinc borate particles using seed crystals. J. Cryst. Growth 2009, 311, 1545–1552. [Google Scholar] [CrossRef]
- Gönen, M. Nanosized Zinc Borate Production. Ph.D. Thesis, İzmir Institute of Technology, İzmir, Turkey, 2009. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.632.5204&rep=rep1&type=pdf (accessed on 16 January 2019).
- Kilinc, M.; Çakal, G.; Yesil, S.; Bayram, G.; Eroglu, I.; Özkar, S. Scale-up synthesis of zinc borate from the reaction of zinc oxide and boric acid in aqueous medium. J. Cryst. Growth 2010, 312, 3361–3366. [Google Scholar] [CrossRef]
- Çakal, G.Ö. Production of fine zinc borate in industrial scale. Chem. Ind. Chem. Eng. Q. 2012, 18, 547–553. [Google Scholar] [CrossRef]
- Burns, P.C.; Hawthorne, F.C. Hydrogen bonding in colemanite: An X-ray and structure-energy study. Can. Mineral. 1993, 31, 297–304. [Google Scholar]
- Corbel, G.; Suard, E.; Emery, J.; Leblanc, M. OH-F disorder in non-centrosymmetric Zn2(BO3)(OH)0.75F0.25: Ab initio structure determination and NMR study; comparison with tridymite and fluoride borates. J. Alloy. Compd. 2000, 305, 49–57. [Google Scholar] [CrossRef]
- Myhren, A.J.; Nelson, E.W. Manufacture of Zinc Borate. U.S. Patent 2,405,366, 6 August 1946. [Google Scholar]
- Harrison, W.T.; Gier, T.E.; Stucky, G.D. The synthesis and ab initio structure determination of Zn4O(BO3)2, a microporous zincoborate constructed of “fused” subunits of three- and five-membered rings. Angew. Chem. Int. Ed. Engl. 1993, 32, 724–726. [Google Scholar] [CrossRef]
- Anonymous. Zinc Borate. Crown 1944, 33, 27–28. [Google Scholar]
- Shen, K.K.; Kochesfahani, S.H.; Jouffret, F. Boron based flame retardants and flame retardancy. In Fire Retardancy of Polymeric Materials, 2nd ed.; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 207–237. ISBN 978-1-4200-8399-6. [Google Scholar]
- Shen, K.K.; Kochesfahani, S.H.; Jouffret, F. Zinc borates as multifunctional polymer additives. Polym. Adv. Technol. 2008, 19, 469–474. [Google Scholar] [CrossRef]
- Shen, K.K. Overview of flame retardancy and smoke suppressant in flexible PVC. In Proceedings of the Society of Plastics Engineering Vinyltech Conference, Atlanta, GA, USA, 18 October 2006. [Google Scholar]
- Shen, K.K. Zinc borates as multifunctional fire retardants in polyamides. In Proceedings of the 13th Annual BCC Conference on Flame Retardancy, Stamford, CT, USA, 3–5 June 2002. [Google Scholar]
- Shen, K.K. Zinc borate as a flame retardant in halogen-free wire and cable systems. In Plastics Compounding; Edgell Communication: Cleveland, OH, USA, 1988. [Google Scholar]
- Shen, K.K. Zinc borates as multifunctional fire retardants in halogen-free polyolefins. In Proceedings of the 14th Annual BCC Conference on Flame Retardancy, Stamford, CT, USA, 2–4 June 2003. [Google Scholar]
- Shen, K.K.; Schultz, D. Flame retardants. In Rubber Technology—Compounding and Testing for Performance; Dick, J.S., Annicelli, R.A., Eds.; Hanser Publications Inc.: Munich, Germany, 2001; p. 248. [Google Scholar]
- Bourbigot, S.; Le Bras, M.; Leeuwendal, R.M.; Shen, K.K.; Schubert, D.M. Recent advances in the use of zinc borate in flame retardancy of EVA. Polym. Degrad. Stab. 1999, 64, 419–425. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Leeuwendal, R.M.; Shen, K.K.; Schubert, D.M. Zinc borate/metal hydroxide formulation in designing FR-EVA materials. Recent Adv. Flame Retard. Polym. Mater. 1998, 9, 245–261. [Google Scholar]
- Le Bras, M.; Bourbigot, S.; Carpentier, F.; Leeuwendal, R.; Schubert, D. Neuere untersuchungen zum einsatz von zinkboraten in EVA. GAK 1998, 12, 972–982. [Google Scholar]
- Schubert, D.M.; Leeuwendal, R.M. The use of a new zinc borate as a flame retardant synergist in engineering thermoplastics. In Proceedings of the Flame Retardants 1998, London, UK, 3–4 February 1998. [Google Scholar]
- Schubert, D.M. Use of borates as fire retardant synergists in talc-filled polypropylene. In Proceedings of the Fire Retardant Chemical Manufacturers Association, Atlanta, GA, USA, April 1998. [Google Scholar]
- Durin-France, A.; Ferry, L.; Lopez Cuesta, J.-M.; Crespy, A. Magnesium hydroxide/zinc borate/talc compositions as flame-retardants in EVA copolymer. Polym. Int. 2000, 49, 1101–1105. [Google Scholar] [CrossRef]
- Laks, P.E.; Manning, M.J. Mobility of Zinc Borate Wood Composite Preservative; IRG/WP/97-30153; The International Research Group on Wood Preservation: Stockholm, Sweden, 1997. [Google Scholar]
- Laks, P.E.; Manning, M.J. Update on the use of borates as preservatives for wood composites. In The Second International Conference on Wood Protection with Diffusible Preservatives and Pesticides; Forest Products Society: LaGrange, GA, USA, 1997; pp. 62–68. [Google Scholar]
- Kirkpatrick, J.W.; Barnes, H.M. Biocide Treatments for Wood Composites—A Review; IRG/WP 06-40323; The International Research Group on Wood Protection: Stockholm, Sweden, 2006. [Google Scholar]
- Wei, W.-S.; Lu, F.; Qin, D.-C.; An, X. Effects of two kinds of boron-based fungicides on the properties of Bambusa emeiensis bamboo scrimber. J. Beijing For. Univ. 2012, 34, 111–115. [Google Scholar]
- Han, G.; Manning, M.; Cheng, W.; Pierre, E.; Wasylciw, W. Performance of zinc borate-treated oriented structural straw board against mold fungi, decay fungi and termites—A preliminary trial. BioResources 2012, 7, 2986–2995. [Google Scholar]
- Evans, P.D.; Lube, V.; Averdunk, H.; Limaye, A.; Turner, M.; Kingston, A.; Senden, T.J. Visualizing the microdistribution of zinc borate in oriented strand board using X-Ray microcomputed tomography and SEM-EDX. J. Compos. 2015, 630905. [Google Scholar] [CrossRef]
- Evans, P.D.; Miesner, M.; Rogerson, D. Machined tapers reduce the differential edge swelling of oriented strand board exposed to water. Compos. B Eng. 2013, 50, 15–21. [Google Scholar] [CrossRef]
- Schubert, D.; Manning, M.; Braiotta, G.; Harrower, S. Hydrolysis of zinc borate as a latent source of boric acid. Manuscript in preparation.
- Schubert, D.M. Multifunctional zinc borate-based anticorrosive pigment. Mod. Paint Coat. 1992, 82, 42–45. [Google Scholar]
- Jackson, W.M., II. Boroflux (zinc borate) low cost flux systems: Reduce the firing temperature of most ceramic bodies to cone 01. Ceram. Eng. Sci. Proc. 1989, 10, 99–108. [Google Scholar]
- Noirot, M.D. Formulating porcelain bodies with borax auxiliary flux. In Ceramic Engineering and Science Proceedings; Carty, W.B., Ed.; American Ceramic Society: Westerville, OH, USA, 2002. [Google Scholar] [CrossRef]





| Q (B2O3/ZnO) | Resolved Oxide Formula | Structural Formula | Industrial Product | Reference |
|---|---|---|---|---|
| 0.19 | 16ZnO∙3B2O3∙3H2O | Zn8(BO3)3O2(OH)3 | [6] | |
| 0.25 | 12ZnO∙3B2O3∙H2O | Zn6O(OH)(BO3)3 | [7,8] | |
| 0.25 | 4ZnO∙B2O3∙H2O | Zn2(BO3)OH | yes | [9,10,11] |
| 0.83 | 6ZnO∙5B2O3∙3H2O | unknown | [12] | |
| 1.00 | ZnO∙B2O3∙∼1.12H2O | Zn(H2O)[B2O4]∼0.12H2O | [13] | |
| 1.00 | 3ZnO∙3B2O3∙5H2O | unknown | yes | |
| 1.50 | 2ZnO∙3B2O3∙3H2O | Zn[B3O4(OH)3] | yes | [12,14,15] |
| 1.50 | 2ZnO∙3B2O3∙7H2O | Zn[B3O3(OH)5]∙H2O | yes | [16] |
| 1.50 | 2ZnO∙3B2O3∙7.5H2O | unknown | [12] | |
| 1.67 | 3ZnO∙5B2O3∙14H2O | Zn3[B5O6(OH)6]∙8H2O a | yes | [17] |
| 3.00 | ZnO∙3B2O3∙4H2O | Zn(H2O)4[B6O10] | [18] | |
| 5.00 | ZnO∙5B2O3∙4.5H2O | unknown | [12] | |
| 6.00 | ZnO∙6B2O3∙5H2O | ZnB12O14(OH)10 | [19] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, D.M. Hydrated Zinc Borates and Their Industrial Use. Molecules 2019, 24, 2419. https://doi.org/10.3390/molecules24132419
Schubert DM. Hydrated Zinc Borates and Their Industrial Use. Molecules. 2019; 24(13):2419. https://doi.org/10.3390/molecules24132419
Chicago/Turabian StyleSchubert, David M. 2019. "Hydrated Zinc Borates and Their Industrial Use" Molecules 24, no. 13: 2419. https://doi.org/10.3390/molecules24132419
APA StyleSchubert, D. M. (2019). Hydrated Zinc Borates and Their Industrial Use. Molecules, 24(13), 2419. https://doi.org/10.3390/molecules24132419