Recent Advances in Conjugated Graft Copolymers: Approaches and Applications
Abstract
:1. Introduction
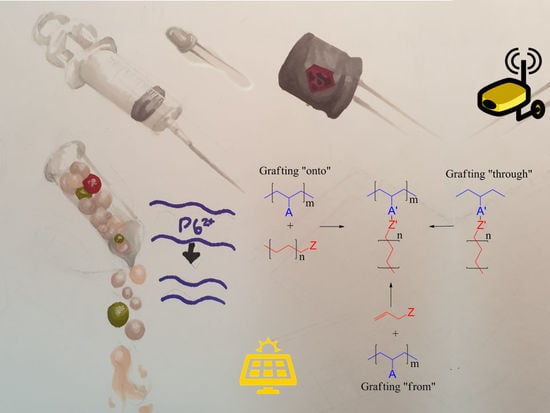
- Grafting “through”—typically includes anionic, free radical polymerisation and ring-opening metathesis, and it is polymerisation from macromonomers. If graft copolymers are produced via this approach, each repeat unit of the copolymer main chain has polymeric side chains, but the distance between them may differ depending on the methods that were used. As an example, in ring-opening metathesis where polynorbornene is used as a backbone, there are four carbon atoms between side chains, and with the use of polymethacrylate there are only two carbon atoms.
- Grafting “onto”—in this method backbone and side chains are prepared separately. This approach can be associated with two potential issues—limited grafting density and how to remove unreacted side chains.
- Grafting “from” allows high grafting density and long backbones to be obtained; in this category, reversible deactivation radical polymerisation, atom transfer radical and reversible nitroxide-mediated polymerisation can be used. These structures could potentially be used in drug delivery systems, as super-soft elastomers, surfactants, lubricants, stimuli-responsive materials, etc. [2].
2. Recent Developments and Applications of Graft Copolymers
2.1. Basic Research on Graft Copolymers
2.2. Graft Conducting Polymer Hydrogels
2.3. Graft Copolymers for Sensing Applications
2.4. Graft Copolymers for Optoelectronic Applications
2.5. Graft Copolymers for Specialised Applications
3. Summary and Future Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APS | ammonium peroxydisulphate |
| AT | aniline tetramer |
| BHJ | bulk hetero-junction |
| CuAAC | copper-catalysed azide–alkyne cycloaddition |
| IPN | interpenetrating polymer network |
| IR | infrared |
| NMR | nuclear magnetic resonance |
| OFET | organic field-effect transistor |
| OSC | organic solar cell |
| P3HT | poly(3-hexylthiophene) |
| PAM | polyacrylamide |
| PANI | polyaniline |
| PANI-g-PSMA | polyaniline-graft-poly(styrene-alt-maleic anhydride) |
| PCE | power conversion efficiency |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| PEG | poly(ethylene glycol) |
| PMMA | poly(methyl methacrylate) |
| PPy | polypyrrole |
| PS | polystyrene |
| PSMA | poly(styrene-alt-maleic anhydride) |
| PTh | polythiophene |
| PVC | poly(vinyl chloride) |
| QCS | quaternised chitosan |
| QCS-g-PANI | quaternised chitosan-graft-polyaniline |
| THF | tetrahydrofuran |
| XG | xanthan gum |
References
- Salhi, F.; Collard, D.M. π-stacked conjugated polymers: The influence of paracyclophane π-stacks on the redox and optical properties of a new class of broken conjugated polythiophenes. Adv. Mater. 2003, 8, 81–85. [Google Scholar] [CrossRef]
- Olszewski, M.; Xie, G.; Matyjaszewski, K.; Sheiko, S.S.; Martinez, M.R. Molecular bottlebrushes as novel materials. Biomacromolecules 2018, 20, 27–54. [Google Scholar]
- Bousquet, A.; Awada, H.; Hiorns, R.C.; Dagron-Lartigau, C.; Billon, L. Conjugated-polymer grafting on inorganic and organic substrates: A new trend in organic electronic materials. Prog. Polym. Sci. 2014, 39, 1847–1877. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Yang, D.; Hu, J.; Zhang, X.; Huang, X. Well-defined graft copolymers: From controlled synthesis to multipurpose applications. Chem. Soc. Rev. 2011, 40, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Strover, L.T.; Malmström, J.; Travas-Sejdic, J. Graft copolymers with conducting polymer backbones: A versatile route to functional materials. Chem. Rec. 2016, 16, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Yassar, A.; Miozzo, L.; Gironda, R.; Horowitz, G. Rod-coil and all-conjugated block copolymers for photovoltaic applications. Prog. Polym. Sci. 2013, 38, 791–844. [Google Scholar] [CrossRef]
- Abd El-Salam, H.M.; Kamal, E.H.M.; Ibrahim, M.S. Synthesis and characterization of chitosan-grafted-poly(2-hydroxyaniline) microstructures for water decontamination. J. Polym. Environ. 2017, 25, 973–982. [Google Scholar] [CrossRef]
- Heydari, M.; Moghadam, P.N.; Fareghi, A.R.; Bahram, M.; Movagharnezhad, N. Synthesis of water-soluble conductive copolymer based on polyaniline. Polym. Adv. Technol. 2015, 26, 250–254. [Google Scholar] [CrossRef]
- Hatamzadeh, M.; Mohammad-Rezaei, R.; Jaymand, M. Chemical and electrochemical grafting of polypyrrole onto thiophene-functionalized polystyrene macromonomer. Mater. Sci. Semicond. Process. 2015, 31, 463–470. [Google Scholar] [CrossRef]
- Massoumi, B.; Mohammad-Rezaei, R.; Jaymand, M. Chemical and electrochemical grafting of polyaniline onto poly(vinyl chloride): Synthesis, characterization, and materials properties. Polym. Adv. Technol. 2016, 27, 1056–1063. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymer hydrogels. Chem. Pap. 2017, 71, 269–291. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Jarosz, T.; Zak, J.K.; Lapkowski, M.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Bednarczyk-Cwynar, B. Advancing the delivery of anticancer drugs: Conjugated polymer/triterpenoid composite. Acta Biomater. 2015, 19, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ge, J.; Ma, P.X.; Guo, B. Injectable conducting interpenetrating polymer network hydrogels from gelatin-graft-polyaniline and oxidized dextran with enhanced mechanical properties. RSC Adv. 2015, 5, 92490–92498. [Google Scholar] [CrossRef]
- Pandey, S.; Ramontja, J. Rapid, facile microwave-assisted synthesis of xanthan gum grafted polyaniline for chemical sensor. Int. J. Biol. Macromol. 2016, 89, 89–98. [Google Scholar] [CrossRef]
- Bicak, T.C.; Gicevičius, M.; Gokoglan, T.C.; Yilmaz, G.; Ramanavicius, A.; Toppare, L.; Yagci, Y. Simultaneous and sequential synthesis of polyaniline-g-poly(ethylene glycol) by combination of oxidative polymerization and cuaac click chemistry: A water-soluble instant response glucose biosensor material. Macromolecules 2017, 50, 1824–1831. [Google Scholar] [CrossRef]
- Molina, B.G.; Bendrea, A.D.; Cianga, L.; Armelin, E.; Del Valle, L.J.; Cianga, I.; Alemán, C. The biocompatible polythiophene: G-polycaprolactone copolymer as an efficient dopamine sensor platform. Polym. Chem. 2017, 8, 6112–6122. [Google Scholar] [CrossRef]
- Molina, B.G.; Cianga, L.; Bendrea, A.-D.D.; Cianga, I.; del Valle, L.J.; Estrany, F.; Alemán, C.; Armelin, E.; Estrany, F. Amphiphilic polypyrrole-poly(Schiff base) copolymers with poly(ethylene glycol) side chains: Synthesis, properties and applications. Polym. Chem. 2018, 9, 4218–4232. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Tarasova, E.V.; Vorobiov, V.K.; Kasatkin, I.A.; Mikli, V.; Sokolova, M.P.; Bobrova, N.V.; Vassiljeva, V.; Krumme, A.; Yakimanskiy, A.V. Electroconductive fibrous mat prepared by electrospinning of polyacrylamide-g-polyaniline copolymers as electrode material for supercapacitors. J. Mater. Sci. 2019, 54, 4859–4873. [Google Scholar] [CrossRef]
- Wang, J.; Higashihara, T. Synthesis of all-conjugated donor-acceptor block copolymers and their application in all-polymer solar cells. Polym. Chem. 2013, 4, 5518–5526. [Google Scholar] [CrossRef]
- Wang, J.; Ueda, M.; Higashihara, T. Synthesis of all-conjugated donor-acceptor-donor ABA-type triblock copolymers via Kumada catalyst-transfer polycondensation. ACS Macro Lett. 2013, 2, 506–510. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Mizobe, T.; Ueda, M.; Chen, W.C.; Higashihara, T. Synthesis and characterization of all-conjugated graft copolymers comprised of n-type or p-type backbones and poly(3-hexylthiophene) side chains. Macromolecules 2013, 46, 1783–1793. [Google Scholar] [CrossRef]
- Wang, J.; Ueda, M.; Higashihara, T. Synthesis and morphology of all-conjugated donor-acceptor block copolymers based on poly(3-hexylthiophene) and poly(naphthalene diimide). J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1139–1148. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Higashihara, T.; Chen, W.C. All-conjugated donor–acceptor graft/block copolymers as single active components and surfactants in all-polymer solar cells. Microsyst. Technol. 2017, 23, 1183–1189. [Google Scholar] [CrossRef]
- Heinrich, C.D.; Thelakkat, M. Poly-(3-hexylthiophene) bottlebrush copolymers with tailored side-chain lengths and high charge carrier mobilities. J. Mater. Chem. C 2016, 4, 5370–5378. [Google Scholar] [CrossRef] [Green Version]
- Van As, D.; Subbiah, J.; Jones, D.J.; Wong, W.W.H. Controlled synthesis of well-defined semiconducting brush polymers. Macromol. Chem. Phys. 2016, 217, 403–413. [Google Scholar] [CrossRef]
- Jarosz, T.; Gebka, K.; Kepska, K.; Lapkowski, M.; Ledwon, P.; Nitschke, P.; Stolarczyk, A. Investigation of the effects of non-conjugated co-grafts on the spectroelectrochemical and photovoltaic properties of novel conjugated graft copolymers based on poly(3-hexylthiophene). Polymers 2018, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, T.; Kepska, K.; Ledwon, P.; Procek, M.; Domagala, W.; Stolarczyk, A. Poly(3-hexylthiophene) grafting and molecular dilution: study of a class of conjugated graft copolymers. Polymers 2019, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, B.; Jaymand, M. Chemical and electrochemical grafting of polythiophene onto poly(methyl methacrylate), and its electrospun nanofibers with gelatin. J. Mater. Sci. Mater. Electron. 2016, 27, 12803–12812. [Google Scholar] [CrossRef]
- Mohammad-Rezaei, R.; Massoumi, B.; Abbasian, M.; Eskandani, M.; Jaymand, M. Electrically conductive adhesive based on novolac-grafted polyaniline: Synthesis and characterization. J. Mater. Sci. Mater. Electron. 2019, 30, 2821–2828. [Google Scholar] [CrossRef]
- Timur, S.; Aydindogan, E.; Yagci, Y.; Arslan, M.; Barlas, F.B. Gold nanoparticle conjugated poly(p-phenylene-β-cyclodextrin)-graft-poly(ethylene glycol) for theranostic applications. J. Appl. Polym. Sci. 2018, 136, 47250. [Google Scholar]
- Senisik, A.M.; Arican, H.A.; Yagci, Y.; Demirkol, D.O.; Timur, S.; Coskunol, H.; Geyik, C.; Guler, B.; Akbulut, H.; Barlas, F.B. Poly(p-phenylene) with poly(ethylene glycol) chains and amino groups as a functional platform for controlled drug release and radiotherapy. Macromol. Biosci. 2016, 16, 730–737. [Google Scholar]
- Cabuk, M.; Yavuz, M.; Unal, H.I. Colloidal, electrorheological, and viscoelastic properties of polypyrrole-graft-chitosan biodegradable copolymer. J. Intell. Mater. Syst. Struct. 2015, 26, 1799–1810. [Google Scholar] [CrossRef]
- Abd El-Salam, H.M.; Kamal, E.H.M.; Ibrahim, M.S. Cleaning of wastewater from total coliform using chitosan-grafted-poly(2-methylaniline). J. Polym. Environ. 2018, 26, 3412–3421. [Google Scholar] [CrossRef]
- Abd El-Salam, H.M.; Mohamed, R.A.; Shokry, A. Preparation and characterization of novel and selective polyacrylamide-graft-poly(2-methoxyaniline) adsorbent for lead removal. Polym. Bull. 2018, 75, 3189–3210. [Google Scholar] [CrossRef]
- Babaladimath, G.; Vishalakshi, B.; Nandibewoor, S.T. Electrical conducting Xanthan Gum-graft-polyaniline as corrosion inhibitor for aluminum in hydrochloric acid environment. Mater. Chem. Phys. 2018, 205, 171–179. [Google Scholar] [CrossRef]























| Copolymer | Copolymer Structural Identification | Study of Copolymer Molecular Weight | Quantitative Composition of the Copolymer | Literature |
|---|---|---|---|---|
| Poly(2-hydroxyaniline)-co-chitosan | IR | No | No | [7] |
| PANI-graft-PSMA | IR | No | No | [8] |
| PS-graft-PPy | IR | GPC | Yes | [9] |
| PANI-graft-PVC | IR | No | No | [10] |
| QCS-graft-PANI | IR, gravimetry | No | Yes | [11] |
| Chitosan-graft-PANI | IR | No | No | [12] |
| Dextran-graft-(aniline tetramer)-graft-(4-formylbenzoic acid) | IR, NMR | No | No | [15] |
| (Oxidised hyaluronic acid)-graft-AT | IR | No | No | [16] |
| Gelatine-graft-PANI | IR | No | No | [18] |
| XG-graft-PANI | IR, gravimetry | No | No | [19] |
| PEG-graft-PANI | IR, NMR | No | No | [20] |
| PTh-graft-PCL | IR | No | No | [21] |
| PPy-poly(Schiff base) copolymers-graft-PEG | IR | No | No | [22] |
| PAM-graft-PANI | IR | No | No | [23] |
| Conjugated cores-graft-P3HT | Prior works | SEC | n/a | [28] |
| PS-graft-P3HT | NMR, IR | MALDI-TOF, SEC | No | [29] |
| PolyNB-graft-P3HT | NMR | MALDI-TOF, GPC | No | [30] |
| Polysiloxanes-graft-PEG-graft-P3HT | NMR, IR | No | Semi | [31] |
| PANI-graft-novolac | NMR (macromonomer only), IR | No | No | [34] |
| Poly(p-phenylene-β-cyclodextrin)-graft-PEG | Prior works | No | No | [35] |
| Poly(p-phenylene)-graft-PEG | Prior works | GPC | No | [36] |
| Chitosan-graft-PPy | Prior works, Elemental analysis | No | No | [37] |
| Chitosan-graft-poly(2-methylaniline) | IR | No | No | [38] |
| PAM-graft-poly(2-methoxyaniline) | IR, NMR, Gravimetry | No | No | [39] |
| PEG-graft-PANI | IR, Gravimetry | No | No | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, T.; Gebka, K.; Stolarczyk, A. Recent Advances in Conjugated Graft Copolymers: Approaches and Applications. Molecules 2019, 24, 3019. https://doi.org/10.3390/molecules24163019
Jarosz T, Gebka K, Stolarczyk A. Recent Advances in Conjugated Graft Copolymers: Approaches and Applications. Molecules. 2019; 24(16):3019. https://doi.org/10.3390/molecules24163019
Chicago/Turabian StyleJarosz, Tomasz, Karolina Gebka, and Agnieszka Stolarczyk. 2019. "Recent Advances in Conjugated Graft Copolymers: Approaches and Applications" Molecules 24, no. 16: 3019. https://doi.org/10.3390/molecules24163019
APA StyleJarosz, T., Gebka, K., & Stolarczyk, A. (2019). Recent Advances in Conjugated Graft Copolymers: Approaches and Applications. Molecules, 24(16), 3019. https://doi.org/10.3390/molecules24163019