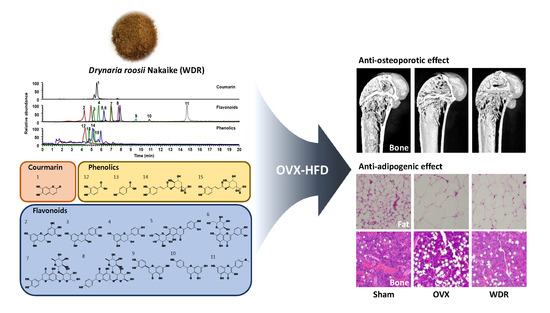
Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of WDR on Bone Loss in HFD-Fed OVX Mice
2.2. Effects of WDR on Osteoclast Differentiation In Vitro
2.3. Effects of WDR on Fat Accumulation in HFD-Fed OVX Mice
2.4. Phytochemical Profiles of WDR
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. WDR Preparation
3.3. Experimental Animal and Diets
3.4. µ-CT Bone Analysis
3.5. Histological Analysis
3.6. Cell Culture and TRAP Assay
3.7. Western Blot Analysis
3.8. UHPLC–MS/MS Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WDR | water extract of D. roosii |
| OVX | ovariectomized |
| HFD | high-fat diet |
| M-CSF | macrophage colony-stimulating factor |
| RANKL | receptor activator of nuclear κ-B ligand |
| BMM | bone marrow-derived macrophage |
References
- Kozakowski, J.; Gietka-Czernel, M.; Leszczynska, D.; Majos, A. Obesity in menopause–our negligence or an unfortunate inevitability? Prz Menopauzalny 2017, 16, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Pae, M.; Baek, Y.; Lee, S.; Wu, D. Loss of ovarian function in association with a high-fat diet promotes insulin resistance and disturbs adipose tissue immune homeostasis. J. Nutr. Biochem. 2018, 57, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizcano, F.; Guzman, G. Estrogen deficiency and the origin of obesity during menopause. Biomed. Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, L.; Chen, X.; Gu, N.; Chen, L.; Zhu, L.; Yang, L.; Pang, L.; Guo, X.; Ji, C.; et al. IL-6 and TNF-alpha induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J. Interferon Cytokine Res. 2014, 34, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Speaker, K.J.; Fleshner, M. Interleukin-1 beta: A potential link between stress and the development of visceral obesity. BMC physiol. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Perez-Llamas, F.; Baraza, J.C.; Garcia-Prieto, M.D.; Fardy, P.S.; Tebar, F.J.; Zamora, S. Body fat distribution in pre-and post-menopausal women: Metabolic and anthropometric variables. J. Nutr. Health Aging 2002, 6, 123–126. [Google Scholar]
- Zhao, G.; Xu, Z.; Shao, Q.; Feng, J.; Xue, J.; Wang, J.; Yang, H.; Li, R.; Li, Y. Confarison of livial and kidney-invigorating traditional Chinese medicine in prevention and treatment of postmenopausal osteoporosis. Chin. J. Osteoporos. 2004, 10, 337–339. [Google Scholar]
- Ruan, X.; Qi, J.; Liu, Y.; Ji, Y.; Chen, B. Effects of traditional Chinese medicine on bone mineral density and femoral neck strength in postmenopausal women. Chin. J. Osteoporos. 2006, 12, 181–184. [Google Scholar]
- Wang, X.; Zhen, L.; Zhang, G.; Wong, M.S.; Qin, L.; Yao, X. Osteogenic effects of flavonoid aglycones from an osteoprotective fraction of Drynaria fortunei—an in vitro efficacy study. Phytomedicine 2011, 18, 868–872. [Google Scholar] [CrossRef]
- Sun, J.S.; Theriault, B.L.; Anderson, G.I. The effect of Gu-Sui-Bu (Drynaria fortunei) on bone cell activity. Am. J. Chin. Med. 2004, 32, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Meng, F.; Xiong, Z.; Li, Y.; Liu, R.; Liu, H. Stimulative activity of Drynaria fortunei (Kunze) J. Sm. extracts and two of its flavonoids on the proliferation of osteoblastic like cells. Pharmazie 2006, 61, 962–965. [Google Scholar] [PubMed]
- Lee, Y.E.; Liu, H.C.; Lin, Y.L.; Liu, S.H.; Yang, R.S.; Chen, R.M. Drynaria fortunei J. Sm. improves the bone mass of ovariectomized rats through osteocalcin-involved endochondral ossification. J. Ethnopharmacol. 2014, 158, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Chang, C.C.; Pang, J.S.; Huang, H.S.; Chou, S.C.; Kao, M.C.; You, H.L. Drynaria fortunei Promoted Angiogenesis Associated With Modified MMP-2/TIMP-2 Balance and Activation of VEGF Ligand/Receptors Expression. Front Pharmacol. 2018, 9, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.C.; Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, M.S. Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br. J. Nutr. 2013, 110, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Qiu, D.; Li, F.; Johnson, F.; Luft, B. Flavonoid of Drynaria fortunei protects against acute renal failure. Phytother. Res. 2005, 19, 422–427. [Google Scholar] [CrossRef]
- Ludgero-Correia, A., Jr.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 2012, 28, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Ohtake, K.; Ehara, N.; Chiba, H.; Nakano, G.; Sonoda, K.; Ito, J.; Uchida, H.; Kobayashi, J. Dietary nitrite reverses features of postmenopausal metabolic syndrome induced by high-fat diet and ovariectomy in mice. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E300–E308. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Yu, X. Fat, Sugar, and Bone Health: A Complex Relationship. Nutrients 2017, 9, 506. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R. A high-fat diet increases body weight and circulating estradiol concentrations but does not improve bone structural properties in ovariectomized mice. Nutr. Res. 2016, 36, 320–327. [Google Scholar] [CrossRef]
- Nilsson, S.; Makela, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Ventura, A.; Marzano, F.; Cavallo, L. Postmenopausal osteoporosis: The role of immune system cells. Clin. Dev. Immunol. 2013, 2013, 575936. [Google Scholar] [CrossRef] [PubMed]
- Yeh, O.C.; Keaveny, T.M. Biomechanical effects of intraspecimen variations in trabecular architecture: A three-dimensional finite element study. Bone 1999, 25, 223–228. [Google Scholar] [CrossRef]
- Perilli, E.; Baleani, M.; Ohman, C.; Fognani, R.; Baruffaldi, F.; Viceconti, M. Dependence of mechanical compressive strength on local variations in microarchitecture in cancellous bone of proximal human femur. J. Biomech. 2008, 41, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Tamura, M.; Maeno, A.; Wakana, S.; Shiroishi, T. Genetic Dissection of Trabecular Bone Structure with Mouse Intersubspecific Consomic Strains. G3 (Bethesda) 2017, 7, 3449–3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negishi-Koga, T.; Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009, 231, 241–256. [Google Scholar] [CrossRef]
- Zamboni, M.; Armellini, F.; Milani, M.P.; De Marchi, M.; Todesco, T.; Robbi, R.; Bergamo-Andreis, I.A.; Bosello, O. Body fat distribution in pre-and post-menopausal women: Metabolic and anthropometric variables and their inter-relationships. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 495–504. [Google Scholar] [PubMed]
- Yamatani, H.; Takahashi, K.; Yoshida, T.; Soga, T.; Kurachi, H. Differences in the fatty acid metabolism of visceral adipose tissue in postmenopausal women. Menopause 2014, 21, 170–176. [Google Scholar] [CrossRef]
- Abildgaard, J.; Pedersen, A.T.; Green, C.J.; Harder-Lauridsen, N.M.; Solomon, T.P.; Thomsen, C.; Juul, A.; Pedersen, M.; Pedersen, J.T.; Mortensen, O.H.; et al. Menopause is associated with decreased whole body fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1227–E1236. [Google Scholar] [CrossRef] [Green Version]
- Lovejoy, J.C.; Champagne, C.M.; De Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. (Lond) 2008, 32, 949–958. [Google Scholar] [CrossRef] [Green Version]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Govindarajan, R.; Sinal, C.J. Bone Marrow Adipose Tissue and Skeletal Health. Curr. Osteoporosis. Rep. 2018, 16, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schurmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018, 19, E360. [Google Scholar] [CrossRef] [PubMed]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Lin, X.H.; Liang, Y.H.; Dong, J.; Guo, D.A.; Ye, M. Comprehensive chemical analysis of the rhizomes of Drynaria fortunei by orthogonal pre-separation and liquid chromatography mass spectrometry. Planta Med. 2014, 80, 330–336. [Google Scholar] [CrossRef]
- Kang, S.N.; Lee, J.S.; Park, J.H.; Cho, J.H.; Park, J.H.; Cho, K.K.; Lee, O.H.; Kim, I.S. In vitro anti-osteoporosis properties of diverse Korean Drynariae rhizoma phenolic extracts. Nutrients 2014, 6, 1737–1751. [Google Scholar] [CrossRef]
- Liang, Y.H.; Ye, M.; Han, J.; Wang, B.R.; Guo, D.A. Lignans and flavonoids from rhizome of Drynaria fortunei. Chin. Tradit. Herbal Drugs 2011, 42, 25–30. [Google Scholar]
- Baek, J.M.; Park, S.H.; Cheon, Y.H.; Ahn, S.J.; Lee, M.S.; Oh, J.; Kim, J.Y. Esculetin attenuates receptor activator of nuclear factor κ-B ligand-mediated osteoclast differentiation through c-Fos/nuclear factor of activated T-cells c1 signaling pathway. Biochem. Biophys. Res. Commun. 2015, 461, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Meijie, L.; Ruihai, W.; Yan, L.; Dong, B.; Jinghua, P.; Hong, L.; Shaojun, W.; Jiaying, W.; Gang, S.; Qing, M.; et al. Effect of esculetin on bone metabolism in ovariectomized rats. J. Tradit. Chin. Med. 2018, 38, 896–903. [Google Scholar] [CrossRef]
- Xu, T.; Wang, L.; Tao, Y.; Ji, Y.; Deng, F.; Wu, X.H. The function of naringin in inducing secretion of osteoprotegerin and inhibiting formation of osteoclasts. Evid. Based Complement. Alternat. Med. 2016, 2016, 8981650. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Noh, A.L.; Zheng, T.; Kang, J.H.; Yim, M. Eriodicyol inhibits osteoclast differentiation and ovariectomy-induced bone loss in vivo. Exp. Cell Res. 2015, 339, 380–388. [Google Scholar] [CrossRef] [PubMed]
- La, V.D.; Tanabe, S.; Grenier, D.J. Naringenin inhibits human osteoclastogenesis and osteoclastic bone resorption. J. Periodontal. Res. 2009, 44, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Xu, W.; Zheng, J.; Shen, P.; Qin, A.; Zhang, S.; Yang, C. Kaempferide prevents titanium particle induced osteolysis by suppressing JNK activation during osteoclast formation. Sci. Rep. 2017, 7, 16665. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Song, H.S.; Kwon, J.E.; Baek, H.J.; Koo, H.J.; Sohn, E.H.; Lee, S.R.; Kang, S.C. Protocatechuic acid attenuates trabecular bone loss in ovariectomized mice. Oxid. Med. Cell. Longev. 2018, 2018, 7280342. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.C.; Lee, C.; Kim, J.Y.; Oh, H.M.; So, H.S.; Lee, M.S.; Rho, M.C.; Oh, J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor κ-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013, 36, 1779–1786. [Google Scholar] [CrossRef]
- Li, F.B.; Sun, X.L.; Ma, J.X.; Zhang, Y.; Zhao, B.; Li, Y.J.; Ma, X.L. Effect of naringin on osteoclast differentiation. Zhongguo Zhong Yao Za Zhi 2015, 40, 308–312. [Google Scholar]
- Ang, E.S.; Yang, X.; Chen, H.; Liu, Q.; Zheng, M.H.; Xu, J. Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-κB and ERK activation. FEBS Lett. 2011, 585, 2755–2762. [Google Scholar] [CrossRef]
- Hirata, M.; Matsumoto, C.; Takita, M.; Miyaura, C.; Inada, M. Naringin Suppresses Osteoclast Formation and Enhances Bone Mass in Mice. J. Health Sci. 2009, 55, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Zhou, L.; Zhao, J.; Liu, Q.; Yang, M.; Tan, R.; Xu, J.; Zhang, G.; Quinn, J.M.; Tickner, J.; et al. Eriodictyol Inhibits RANKL-Induced Osteoclast Formation and Function Via Inhibition of NFATc1 Activity. J. Cell. Physiol. 2016, 231, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Shin, S.H.; Kim, B.J.; Kim, C.H.; Kim, J.H.; Kang, H.M.; Park, B.S.; Kim, I.R. The Effects of Kaempferol-Inhibited Autophagy on Osteoclast Formation. Int. J. Mol. Sci. 2018, 19, E125. [Google Scholar] [CrossRef] [PubMed]
- Wattel, A.; Kamel, S.; Mentaverri, R.; Lorget, F.; Prouillet, C.; Petit, J.P.; Fardelonne, P.; Brazier, M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem. Pharmacol. 2003, 65, 35–42. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.Y.; Cheon, Y.H.; Baek, J.M.; Ahn, S.J.; Yoon, K.H.; Lee, M.S.; Oh, J. Protocatechuic Acid Attenuates Osteoclastogenesis by Downregulating JNK/c-Fos/NFATc1 Signaling and Prevents Inflammatory Bone Loss in Mice. Phytother. Res. 2016, 30, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Wu, T.Y.; Xu, B.B.; Xu, X.Y.; Chen, H.G.; Li, X.Y.; Wang, G. Protocatechuic acid inhibits osteoclast differentiation and stimulates apoptosis in mature osteoclasts. Biomed. Pharmacother. 2016, 82, 399–405. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J. Esculetin Inhibits Adipogenesis and increases antioxidant activity during adipocyte differentiation in 3T3-L1 cells. Prev. Nutr. Food Sci. 2017, 22, 118–123. [Google Scholar]
- Rashid, A.M.; Lu, K.; Yip, Y.M.; Zhang, D. Averrhoa carambola L. peel extract suppresses adipocyte differentiation in 3T3-L1 cells. Food Funct. 2016, 7, 881–892. [Google Scholar] [CrossRef]
- Kim, J.; Lee, I.; Seo, J.; Jung, M.; Kim, Y.; Yim, N.; Bae, K. Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells. Phytother. Res. 2010, 24, 1543–1548. [Google Scholar] [CrossRef]
- Fan, J.; Li, J.; Fan, Q. Naringin promotes differentiation of bone marrow stem cells into osteoblasts by upregulating the expression levels of microRNA-20a and downregulating the expression levels of PPARγ. Mol. Med. Rep. 2015, 12, 4759–4765. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; Amini-Vaughan, Z.; Ribnicky, D.M.; Stephens, J.M. Naringenin inhibits adipogenesis and reduces insulin sensitivity and adiponectin expression in adipocytes. Evid. Based Complement. Alternat. Med. 2013, 2013, 549750. [Google Scholar] [CrossRef] [PubMed]
- Kumkarnjana, S.; Suttisri, R.; Nimmannit, U.; Koobkokkruad, T.; Pattamadilok, C.; Vardhanabhuti, N. Anti-adipogenic effect of flavonoids from Chromolaena odorata leaves in 3T3-L1 adipocytes. J. Integr. Med. 2018, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Piza, A.; An, Y.J.; Kim, D.K.; Lee, S.H.; Kim, J.B.; Choi, J.S.; Lee, S.J. Protocatechuic acid enhances osteogenesis, but inhibits adipogenesis in C3H10T1/2 and 3T3-L1 cells. J. Med. Food 2017, 20, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaew, S. Potential of coffee fruit extract and quinic acid on adipogenesis and lipolysis in 3T3-L1 adipocytes. Kobe J. Med. Sci. 2018, 64, E84–E92. [Google Scholar]
- Poudel, B.; Nepali, S.; Xin, M.; Ki, H.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol. Med. Rep. 2015, 12, 3139–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lee, J. Esculetin, a coumarin derivative, suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes. J. Funct. Foods 2015, 12, 509–515. [Google Scholar] [CrossRef]
- Yang, J.Y.; Della-Fera, M.A.; Hartzell, D.L.; Nelson-Dooley, C.; Hausman, D.B.; Baile, C.A. Esculetin induces apoptosis and inhibits adipogenesis in 3T3-L1 cells. Obesity (Silver Spring) 2006, 14, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Matsumoto, C.; Shibano, M.; Fujimori, K. Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPdelta Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes. Nutrients 2018, 10, E130. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, H.S.; Seo, M.J.; Jeon, H.J.; Kim, K.J.; Lee, B.Y. Kaempferol suppresses lipid accumulation by inhibiting early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct. 2015, 6, 2824–2833. [Google Scholar] [CrossRef]
- Ha, H.; An, H.; Shim, K.S.; Kim, T.; Lee, K.J.; Hwang, Y.H.; Ma, J.Y. Ethanol extract of Atractylodes macrocephala protects bone loss by inhibiting osteoclast differentiation. Molecules 2013, 18, 7376–7388. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Ma, J.Y. Preventive Effects of an UPLC-DAD-MS/MS Fingerprinted Hydroalcoholic Extract of Citrus aurantium in a Mouse Model of Ulcerative Colitis. Planta Med. 2018, 84, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |




| No | tR (min) | Precursor Ion (m/z) | Elemental Composition | Error (ppm) | MS/MS Fragments (m/z) | Identification | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Estimated | Calculated | Adduct | |||||||
| Coumarin | |||||||||
| 1 | 5.5 | 179.0339 | 179.0339 | [M + H]+ | C9H6O4 | 0.169 | 179, 163 | Esculetin * | - |
| Flavonoids | |||||||||
| 2 | 4.2 | 305.0669 | 305.0667 | [M − H]− | C15H14O7 | 0.803 | 305, 219 | (−)-Gallocatechin * | [39] |
| 3 | 5.1 | 289.0721 | 289.0718 | [M − H]− | C15H14O6 | 1.327 | 289, 245, 109 | (−)-Catechin * | - |
| 4 | 5.7 | 289.0721 | 289.0718 | [M − H]− | C15H14O6 | 1.221 | 289, 245, 203, 125 | (−)-Epicatechin * | [38] |
| 5 | 6.2 | 447.0938 | 447.0933 | [M − H]− | C21H20O11 | 1.188 | 447, 327 | Isoorientin * | - |
| 6 | 6.2 | 447.0938 | 447.0933 | [M − H]− | C21H20O11 | 1.188 | 447, 357, 327 | Orientin * | - |
| 7 | 6.9 | 595.1676 | 595.1668 | [M − H]− | C27H32O15 | 1.281 | 595, 459, 151 | Neoeriocitrin | [40] |
| 8 | 7.7 | 579.1725 | 579.1719 | [M − H]− | C27H32O14 | 0.959 | 459, 271 | Naringin * | [40] |
| 9 | 9.4 | 287.0565 | 287.0561 | [M − H]− | C15H12O6 | 1.423 | 287, 151, 135 | Eriodictyol * | [40] |
| 10 | 10.8 | 271.0615 | 271.0612 | [M − H]− | C15H12O5 | 1.193 | 271, 151 | Naringenin | [10] |
| 11 | 14.7 | 299.0563 | 299.0561 | [M − H]− | C16H12O6 | 0.754 | 299, 284 | Kaempferide * | - |
| Phenolics | |||||||||
| 12 | 4.3 | 155.034 | 155.0339 | [M + H]+ | C7H6O4 | 0.589 | 132, 114, 155 | Protocatechuic acid * | [38] |
| 13 | 4.7 | 353.0882 | 353.0878 | [M − H]− | C16H18O9 | 1.006 | 353, 191, 179, 135 | Neochlorogenic acid * | - |
| 14 | 5.1 | 139.0391 | 139.0390 | [M + H]+ | C7H6O3 | 0.665 | 139, 121 | P-hydroxybenzoic acid * | - |
| 15 | 5.2 | 353.0881 | 353.0878 | [M − H]− | C16H18O9 | 0.920 | 191, 179, 135 | Chlorogenic acid * | [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-A.; Hwang, Y.-H.; Kim, T.; Lee, A.; Ha, H. Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet. Molecules 2019, 24, 3051. https://doi.org/10.3390/molecules24173051
Jang S-A, Hwang Y-H, Kim T, Lee A, Ha H. Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet. Molecules. 2019; 24(17):3051. https://doi.org/10.3390/molecules24173051
Chicago/Turabian StyleJang, Seon-A, Youn-Hwan Hwang, Taesoo Kim, Ami Lee, and Hyunil Ha. 2019. "Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet" Molecules 24, no. 17: 3051. https://doi.org/10.3390/molecules24173051
APA StyleJang, S. -A., Hwang, Y. -H., Kim, T., Lee, A., & Ha, H. (2019). Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet. Molecules, 24(17), 3051. https://doi.org/10.3390/molecules24173051