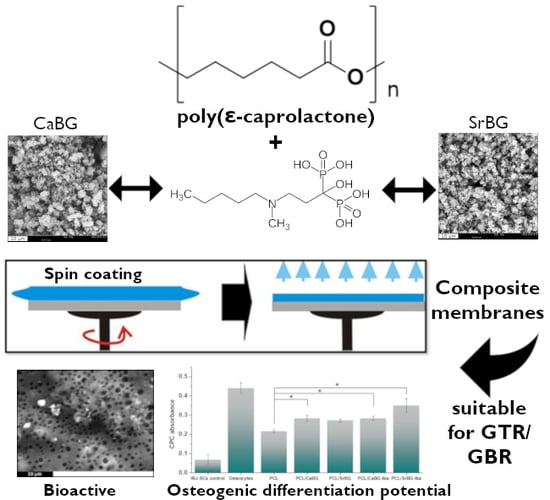
Composite Membranes of Poly(ε-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Bioglasses
Adsorption of Iba on the Bioglasses
2.2. Characterization of Composite PCL Thin Films
2.2.1. Morphological Characterization
2.2.2. Structural Characterization
2.2.3. Thermal Properties
2.2.4. Mechanical Properties
2.2.5. Hydrophilicity
2.2.6. In Vitro Bioactivity
2.2.7. Ion Release
2.2.8. In Vitro Cytocompatibility and Osteogenic Potential
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Mesoporous Ternary Bioglasses
3.3. Synthesis of PCL via Ring Opening Polymerization (ROP)
3.4. Loading of CaBG and SrBG Bioglasses with Ibandronate
3.5. Preparation of PCL Composite Thin Films with Bioglasses
3.6. Characterization
3.6.1. Characterization of CaBG and SrBG
3.6.2. Characterization of the Composite Films
3.7. Cell Studies
3.7.1. Isolation, Cultivation and Genetic Modification of Wharton’s Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs)
3.7.2. Sterilization of the Materials and WJ-SCs Plating
3.7.3. 3-[4,5-Dimethylthiazole-2-yl]-2,5-diphenyltetrazolium Bromide (MTT) Assay
3.7.4. Observation under Fluorescence Microscope
3.7.5. Osteogenic Differentiation—Alizarin Red Staining and CPC Quantification
3.7.6. Statistical Analysis
3.7.7. Ethical Statement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.-L.; Kapila, Y.; Lin, G.-H. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Kerns, D.G. Suppl 1: Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56. [Google Scholar] [CrossRef]
- Murphy, K.G.; Gunsolley, J.C. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects: A systematic review. Ann. Periodontol. 2003, 8, 266–302. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Tempro, P.J.; Nalbandian, J. Colonization of retrieved polytetrafluoroethylene membranes: Morphological and microbiological observations. J. Periodontol. 1993, 64, 162–168. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Lee, E.J.; Teng, S.H.; Jang, T.S.; Wang, P.; Yook, S.W.; Kim, H.E.; Koh, Y.H. Nanostructured poly(ε-caprolactone)-silica xerogel fibrous membrane for guided bone regeneration. Acta Biomater. 2010, 6, 3557–3565. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Ma, J.; Bouma, M.J.; Boerman, O.C.; Chen, Z.; van den Beucken, J.J.J.P.; Jansen, J.A. Incorporation of stromal cell-derived factor-1α in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials 2013, 34, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.; Jun, H.K.; Se, H.O.; Hyun, H.N.; Jin, M.K.; Lee, J.H. Hydrophilized polycaprolactone nanofiber mesh-embedded poly(glycolic-co- lactic acid) membrane for effective guided bone regeneration. J. Biomed. Mater. Res. Part A 2009, 91, 400–407. [Google Scholar]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Park, C.Y.; Bae, J.H.; Ahn, G.; Kim, C.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Shim, J.H.; Huh, J.B. Evaluation of 3D printed PCL/PLGA/β-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-printed polycaprolactone/β-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef] [PubMed]
- Pitaluga, L.H.; Souza, M.T.; Zanotto, E.D.; Romero, M.E.S.; Hatton, P.V. Electrospun F18 bioactive glass/PCL-poly (ε-caprolactone)-membrane for guided tissue regeneration. Materials 2018, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lv, S.; Lu, J.; Jiang, S.; Lin, L. Characterization of polycaprolactone/collagen fibrous scaffolds by electrospinning and their bioactivity. Int. J. Biol. Macromol. 2015, 76, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Huh, J.-B.; Park, J.Y.; Jeon, Y.-C.; Kang, S.S.; Kim, J.Y.; Rhie, J.-W.; Cho, D.-W. Fabrication of blended polycaprolactone/poly (lactic-Co-glycolic acid)/β-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng. Part A 2012, 19, 317–328. [Google Scholar] [CrossRef]
- Chakraborti, M.; Jackson, J.K.; Plackett, D.; Brunette, D.M.; Burt, H.M. Drug intercalation in layered double hydroxide clay: Application in the development of a nanocomposite film for guided tissue regeneration. Int. J. Pharm. 2011, 416, 305–313. [Google Scholar] [CrossRef]
- Rowe, M.J.; Kamocki, K.; Pankajakshan, D.; Li, D.; Bruzzaniti, A.; Thomas, V.; Blanchard, S.B.; Bottino, M.C. Dimensionally stable and bioactive membrane for guided bone regeneration: An in vitro study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 594–605. [Google Scholar] [CrossRef]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef]
- Castro, A.G.B.; Diba, M.; Kersten, M.; Jansen, J.A.; van den Beucken, J.J.J.P.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for guided bone regeneration. Mater. Sci. Eng. C 2018, 85, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Yu, H.S.; Jang, J.H.; Kim, H.W. Bioactivity improvement of poly(ε-caprolactone) membrane with the addition of nanofibrous bioactive glass. Acta Biomater. 2008, 4, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.I.; Caridade, S.G.; Ma, J.; Yu, N.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Asymmetric PDLLA membranes containing bioglass® for guided tissue regeneration: Characterization and in vitro biological behavior. Dent. Mater. 2013, 29, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Frank Walboomers, X.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wu, Y.; Du, Y.; Chen, X.; Lei, B.; Xue, Y.; Ma, P.X. A highly bioactive and biodegradable poly(glycerol sebacate)-silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3222–3233. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Resorbable PCEC/gelatin-bismuth doped bioglass-graphene oxide bilayer membranes for guided bone regeneration. Biomed. Mater. 2019. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Gentile, P.; Martins, M.; Neves, N.M.; Miller, C.; Crawford, A.; Pires, R.A.; Hatton, P.; Reis, R.L. Reinforcement of poly-L-lactic acid electrospun membranes with strontium borosilicate bioactive glasses for bone tissue engineering. Acta Biomater. 2016, 44, 168–177. [Google Scholar] [CrossRef]
- Dehnavi, S.S.; Mehdikhani, M.; Rafienia, M.; Bonakdar, S. Preparation and in vitro evaluation of polycaprolactone/PEG/bioactive glass nanopowders nanocomposite membranes for GTR/GBR applications. Mater. Sci. Eng. C 2018, 90, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ding, Y.; Yu, S.; Yao, Q.; Boccaccini, A.R. Multifunctional chitosan-45S5 bioactive glass-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) microsphere composite membranes for guided tissue/bone regeneration. ACS Appl. Mater. Interfaces 2015, 7, 20845–20854. [Google Scholar] [CrossRef] [PubMed]
- Caridade, S.G.; Merino, E.G.; Martins, G.V.; Luz, G.M.; Alves, N.M.; Mano, J.F. Membranes of poly(dl-lactic acid)/bioglass® with asymmetric bioactivity for biomedical applications. J. Bioact. Compat. Polym. 2012, 27, 429–440. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Nano-hydroxyapatite composite biomaterials for bone tissue engineering—a review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Pan, Y.Z.; Li, L.; He, C. Bin Nanocomposites for bone tissue regeneration. Nanomedicine 2013, 8, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Bramhill, J.; Ross, S.; Ross, G. Bioactive nanocomposites for tissue repair and regeneration: A review. Int. J. Environ. Res. Public Health 2017, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; Ray, S.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [Green Version]
- Huang, J. Chapter 20—design and development of ceramics and glasses. In Biology and Engineering of Stem Cell Niches 2017; Vishwakarma, A., Karp, J.M., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 315–329. [Google Scholar]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef]
- Shahin-Shamsabadi, A.; Hashemi, A.; Tahriri, M.; Bastami, F.; Salehi, M.; Mashhadi Abbas, F. Mechanical, material, and biological study of a PCL/bioactive glass bone scaffold: Importance of viscoelasticity. Mater. Sci. Eng. C 2018, 90, 280–288. [Google Scholar] [CrossRef]
- Mouriño, V.; Vidotto, R.; Cattalini, J.P.; Boccaccini, A.R. Enhancing biological activity of bioactive glass scaffolds by inorganic ion delivery for bone tissue engineering. Curr. Opin. Biomed. Eng. 2019, 10, 23–34. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.-H.; Kim, H.-W.; Salih, V.; Wall, I.B.; Knowles, J.C. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Xiang, Y. Effect of strontium substitution on the structure, ionic diffusion and dynamic properties of 45S5 bioactive glasses. J. Non. Cryst. Solids 2012, 358, 1059–1071. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, K. Strontium eluting graphene hybrid nanoparticles augment osteogenesis in a 3D tissue scaffold. Nanoscale 2015, 7, 2023–2033. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, G.; Luk, K.D.K.; Cheung, K.M.C.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cell. Physiol. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Aimaiti, A.; Maimaitiyiming, A.; Boyong, X.; Aji, K.; Li, C.; Cui, L. Low-dose strontium stimulates osteogenesis but high-dose doses cause apoptosis in human adipose-derived stem cells via regulation of the ERK1/2 signaling pathway. Stem Cell Res. Ther. 2017, 8, 282. [Google Scholar] [CrossRef]
- Denry, I.; Goudouri, O.-M.; Fredericks, D.C.; Akkouch, A.; Acevedo, M.R.; Holloway, J.A. Strontium-releasing fluorapatite glass-ceramic scaffolds: Structural characterization and in vivo performance. Acta Biomater. 2018, 75, 463–471. [Google Scholar] [CrossRef]
- Zhang, H.; Moriyama, Y.; Ayukawa, Y.; Rakhmatia, Y.D.; Tomita, Y.; Yasunami, N.; Koyano, K. Generation and histomorphometric evaluation of a novel fluvastatin-containing poly(lactic-co-glycolic acid) membrane for guided bone regeneration. Odontology 2019, 107, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Abdeslam-Mohamed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernández-Alfaro, F.; Miron, R. Adsorption and release kinetics of growth factors on barrier membranes for guided tissue/bone regeneration: A systematic review. Arch. Oral Biol. 2019, 100, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hum, J.; Boccaccini, A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: A review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Camargo, W.A.; Zinkevich, T.; Grünewald, A.; Detsch, R.; Kabiri, Y.; Kentgens, A.P.M.; Boccaccini, A.R.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G. Hybrid particles derived from alendronate and bioactive glass for treatment of osteoporotic bone defects. J. Mater. Chem. B 2019, 7, 796–808. [Google Scholar] [CrossRef]
- Rosenqvist, K.; Airaksinen, S.; Fraser, S.J.; Gordon, K.C.; Juppo, A.M. Interaction of bioactive glass with clodronate. Int. J. Pharm. 2013, 452, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Kim, H.J.; Park, K.; Song, H.R. 3D printed alendronate-releasing poly(caprolactone) porous scaffolds enhance osteogenic differentiation and bone formation in rat tibial defects. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ezra, A.; Golomb, G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv. Drug Deliv. Rev. 2000, 42, 175–195. [Google Scholar] [CrossRef]
- Fleish, H. Bisphosphonates. Drugs 1991, 42, 919–944. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, J.H.; Abbas, A.A.; Yoon, T.R. Alendronate enhances osteogenic differentiation of bone marrow stromal cells: A preliminary study. Clin. Orthop. Relat. Res. 2009, 467, 3121–3128. [Google Scholar] [CrossRef]
- Long, K.A.; Jackson, J.K.; Yang, C.; Chehroudi, B.; Brunette, D.M.; Burt, H.M. Controlled release of alendronate from polymeric films. J. Biomater. Sci. Polym. Ed. 2009, 20, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Bikiaris, D. Biocompatible nanobioglass reinforced poly(ε-caprolactone) composites synthesized via in situ ring opening polymerization. Polymers 2018, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yamamoto, A. Characteristics and cytocompatibility of biodegradable polymer film on magnesium by spin coating. Colloid. Surface. B 2012, 93, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Jang, Y.S.; Kim, J.H.; Lee, M.H. Improvement of osteogenesis by a uniform PCL coating on a magnesium screw for biodegradable applications. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chlanda, A.; Oberbek, P.; Heljak, M.; Kijeńska-Gawrońska, E.; Bolek, T.; Gloc, M.; John, Ł.; Janeta, M.; Woźniak, M.J. Fabrication, multi-scale characterization and in-vitro evaluation of porous hybrid bioactive glass polymer-coated scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 94, 516–523. [Google Scholar] [CrossRef]
- Alani, A.; Knowles, J.C.; Chrzanowski, W.; Ng, Y.L.; Gulabivala, K. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass-polycaprolactone-based composite for use as a root canal obturation material. Dent. Mater. 2009, 25, 400–410. [Google Scholar] [CrossRef]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Cunningham, E.; Lennon, A.; McCarthy, H.O.; Buchanan, F.; Clarke, S.A.; Dunne, N. Effects of poly (ε-caprolactone) coating on the properties of three-dimensional printed porous structures. J. Mech. Behav. Biomed. Mater. 2017, 70, 68–83. [Google Scholar] [CrossRef]
- Fiedler, T.; Videira, A.C.; Bártolo, P.; Strauch, M.; Murch, G.E.; Ferreira, J.M.F. On the mechanical properties of PLC-bioactive glass scaffolds fabricated via BioExtrusion. Mater. Sci. Eng. C 2015, 57, 288–293. [Google Scholar] [CrossRef]
- Mourão, H.A.J.L.; Lopes, O.F.; Ribeiro, C.; Mastelaro, V.R. Rapid hydrothermal synthesis and pH-dependent photocatalysis of strontium titanate microspheres. Mater. Sci. Semicond. Process. 2015, 30, 651–657. [Google Scholar] [CrossRef]
- Yun, H.; Kim, S.; Lee, S.; Song, I. Synthesis of high surface area mesoporous bioactive glass nanospheres. Mater. Lett. 2010, 64, 1850–1853. [Google Scholar] [CrossRef]
- Khamsehashari, N.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol-gel method. Mater. Chem. Phys. 2018, 205, 283–291. [Google Scholar] [CrossRef]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non. Cryst. Solids 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Characterization, in vitro bioactivity and biological studies of sol-gel synthesized SrO substituted 58S bioactive glass. Ceram. Int. 2017, 43, 14880–14890. [Google Scholar] [CrossRef]
- Bizari, D.; Rabiee, M.; Moztarzadeh, F.; Tahriri, M.; Alavi, S.H.; Masaeli, R. Synthesis, characterization and biological evaluation of sol-gel derived nanomaterial in the ternary system 64% SiO2 - 31% CaO - 5% P2O5 as a bioactive glass: In vitro study. Ceram.-Silikaty 2013, 57, 201–209. [Google Scholar]
- Luz, G.M.; Mano, J.F. Preparation and characterization of bioactive glass nanoparticles prepared by sol-gel for biomedical applications. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Łączka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. Bioactivity and osteoinductivity of glasses and glassceramics and their material determinants. Ceram. Int. 2016, 42, 14313–14325. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, D.; Li, N.; Wen, J.; Jiang, X.; Liu, C.; Li, Y. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 2016, 6, 19361. [Google Scholar] [CrossRef]
- Fujikura, K.; Karpukhina, N.; Kasuga, T.; Brauer, D.S.; Hill, R.G.; Law, R.V. Influence of strontium substitution on structure and crystallisation of Bioglass® 45S5. J. Mater. Chem. 2012, 22, 7395–7402. [Google Scholar] [CrossRef]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol–gel-derived mesoporous bioactive glass (MBG) powder. J. Sol-Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Suzuki, K.; Ikari, K.; Imai, H. Synthesis of silica nanoparticles having a well-ordered mesostructure using a double surfactant system. J. Am. Chem. Soc. 2004, 126, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Z.; Quan, G.; Peng, X.; Pan, X.; Wang, R.; Xu, Y.; Li, G.; Wu, C. In vitro and in vivo evaluation of ordered mesoporous silica as a novel adsorbent in liquisolid formulation. Int. J. Nanomedicine 2012, 7, 199. [Google Scholar] [PubMed]
- Tian, L.; Peng, C.; Shi, Y.; Guo, X.; Zhong, B.; Qi, J.; Wang, G.; Cai, Q.; Cui, F. Effect of mesoporous silica nanoparticles on dentinal tubule occlusion: An in vitro study using SEM and image analysis. Dent. Mater. J. 2014, 33, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.; Dong, X.; Zhang, L.; Zeng, H. A mesoporous bioactive glass/polycaprolactone composite scaffold and its bioactivity behavior. J. Biomed. Mater. Res. Part A 2008, 84, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous bioactive glasses: Relevance of their porous structure compared to that of classical bioglasses. Biomed. Glas. 2015. [Google Scholar] [CrossRef]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The incorporation of strontium to improve bone-regeneration ability of mesoporous bioactive glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, W.; Chen, X.; Li, Y.; Liang, Q. The effects of Sr concentration on physicochemical properties, bioactivity and biocompatibility of sub-micron bioactive glasses spheres. Adv. Powder Technol. 2017, 28, 2713–2722. [Google Scholar] [CrossRef]
- Shahrabi, S.; Hesaraki, S.; Moemeni, S.; Khorami, M. Structural discrepancies and in vitro nanoapatite formation ability of sol-gel derived glasses doped with different bone stimulator ions. Ceram. Int. 2011, 37, 2737–2746. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Xiao, Y. Mesoporous bioactive glasses as drug delivery and bone tissue regeneration platforms. Ther. Deliv. 2011, 2, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Srisubut, S.; Teerakapong, A.; Vattraphodes, T.; Taweechaisupapong, S. Effect of local delivery of alendronate on bone formation in bioactive glass grafting in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 104, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, D.; Weng, W.; Huang, Q.; Zhang, X.; Wen, J.; Wu, J.; Jiang, X. Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2015, 30, 391–396. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Vallet-Regí, M. Functionalizing mesoporous bioglasses for long-term anti-osteoporotic drug delivery. Chem. A Eur. J. 2010, 16, 10879–10886. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, G.; Magrone, D.; Castaldi, G. Polymorphic Forms of Ibandronate Sodium and Processes for Preparation Thereof. International Patent Application No. PCT/EP2008/064401, 30 April 2009. [Google Scholar]
- Simon, D.; Holland, A.; Shanks, R. Poly (caprolactone) thin film preparation, morphology, and surface texture. J. Appl. Polym. Sci. 2007, 103, 1287–1294. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Vozzi, G.; Whulanza, Y.; Seggiani, M.; Fantauzzi, V.; Orsini, G.; Ahluwalia, A. Tuning polycaprolactone-carbon nanotube composites for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2012, 32, 152–159. [Google Scholar] [CrossRef]
- Degner, J.; Singer, F.; Cordero, L.; Boccaccini, A.R.; Virtanen, S. Electrochemical investigations of magnesium in DMEM with biodegradable polycaprolactone coating as corrosion barrier. Appl. Surf. Sci. 2013, 282, 264–270. [Google Scholar] [CrossRef]
- Dziadek, M.; Zagrajczuk, B.; Ziabka, M.; Dziadek, K.; Cholewa-Kowalska, K. The role of solvent type, size and chemical composition of bioactive glass particles in modulating material properties of poly(ε-caprolactone) based composites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 90–99. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of surface functionalization of halloysite nanotubes on synthesis and thermal properties of poly(ε-caprolactone). J. Mater. Sci. 2018, 53, 6519–6541. [Google Scholar] [CrossRef]
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Synthesis and electrospinning of ε-polycaprolactone-bioactive glass hybrid biomaterials via a sol-gel process. Langmuir 2010, 26, 18340–18348. [Google Scholar] [CrossRef]
- Larrañaga, A.; Sarasua, J.-R. Effect of bioactive glass particles on the thermal degradation behaviour of medical polyesters. Polym. Degrad. Stab. 2013, 98, 751–758. [Google Scholar] [CrossRef]
- Diamanti, E.; Sarasua, J.R. Effects of bioactive glass particles on the mechanical and thermal behavior of poly (ε-caprolactone). Macromol. Symp. 2012, 321–322, 25–29. [Google Scholar] [CrossRef]
- Larrañaga, A.; Petisco, S.; Sarasua, J.R. Improvement of thermal stability and mechanical properties of medical polyester composites by plasma surface modification of the bioactive glass particles. Polym. Degrad. Stab. 2013, 98, 1717–1723. [Google Scholar] [CrossRef]
- Dziadek, M.; Pawlik, J.; Menaszek, E.; Stodolak-Zych, E.; Cholewa-Kowalska, K. Effect of the preparation methods on architecture, crystallinity, hydrolytic degradation, bioactivity, and biocompatibility of PCL/bioglass composite scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1580–1593. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Stevens, M.M.; Woodruff, M.A. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication 2013, 5. [Google Scholar] [CrossRef]
- Goel, A.; Rajagopal, R.R.; Ferreira, J.M.F. Influence of strontium on structure, sintering and biodegradation behaviour of CaO-MgO-SrO-SiO2-P2O5-CaF2 glasses. Acta Biomater. 2011, 7, 4071–4080. [Google Scholar] [CrossRef]
- Bang, L.T.; Ramesh, S.; Purbolaksono, J.; Ching, Y.C.; Long, B.D.; Chandran, H.; Othman, R. Effects of silicate and carbonate substitution on the properties of hydroxyapatite prepared by aqueous co-precipitation method. Mater. Des. 2015, 87, 788–796. [Google Scholar] [CrossRef]
- Lao, J.; Jallot, E.; Nedelec, J.-M. Strontium-delivering glasses with enhanced bioactivity: A new biomaterial for antiosteoporotic applications? Chem. Mater. 2008, 20, 4969–4973. [Google Scholar] [CrossRef]
- Özarslan, A.C.; Yücel, S. Fabrication and characterization of strontium incorporated 3-D bioactive glass scaffolds for bone tissue from biosilica. Mater. Sci. Eng. C 2016, 68, 350–357. [Google Scholar] [CrossRef]
- Hesaraki, S.; Gholami, M.; Vazehrad, S.; Shahrabi, S. The effect of Sr concentration on bioactivity and biocompatibility of sol-gel derived glasses based on CaO-SrO-SiO2-P2O5 quaternary system. Mater. Sci. Eng. C 2010, 30, 383–390. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Chang, J.; Miron, R.J.; Shi, B.; Yi, S.; Wu, C. Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B 2013, 1, 5711–5722. [Google Scholar] [CrossRef]
- Murphy, S.; Wren, A.W.; Towler, M.R.; Boyd, D. The effect of ionic dissolution products of Ca-Sr-Na-Zn-Si bioactive glass on in vitro cytocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadou, I.; Nianias, N.; Hoppe, A.; Terzopoulou, Z.; Bikiaris, D.; Will, J.; Hum, J.; Roether, J.A.; Detsch, R.; Boccaccini, A.R. Evaluation of silica-nanotubes and strontium hydroxyapatite nanorods as appropriate nanoadditives for poly(butylene succinate) biodegradable polyester for biomedical applications. Compos. Part B Eng. 2014, 60, 49–59. [Google Scholar] [CrossRef]
- Koliakou, I.; Gounari, E.; Nerantzaki, M.; Pavlidou, E.; Bikiaris, D.; Kaloyianni, M.; Koliakos, G. Differentiation capacity of monocyte-derived multipotential cells on nanocomposite poly (ε-caprolactone)-based thin films. Tissue Eng. Regen. Med. 2019, 16, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Khambatta, F.B.; Warner, F.; Russell, T.; Stein, R.S. Small-angle X-ray and light scattering studies of the morphology of blends of poly (ε-caprolactone) with poly(vinyl chloride). J. Polym. Sci. Pol. Phys. 1976. [Google Scholar] [CrossRef]
- Tzetzis, D.; Tsongas, K.; Mansour, G. Determination of the mechanical properties of epoxy silica nanocomposites through FEA-supported evaluation of ball indentation test results. Mater. Res. 2017, 20, 1571–1578. [Google Scholar] [CrossRef]
- Mansour, G.; Tzetzis, D.; Bouzakis, K.D. A nanomechanical approach on the measurement of the elastic properties of epoxy reinforced carbon nanotube nanocomposites. Tribol. Ind. 2013, 35, 190–199. [Google Scholar]
- Tzetzis, D.; Mansour, G.; Tsiafis, I.; Pavlidou, E. Nanoindentation measurements of fumed silica epoxy reinforced nanocomposites. J. Reinf. Plast. Compos. 2013, 32, 160–173. [Google Scholar] [CrossRef]
- Mansour, G.; Tzetzis, D. Nanomechanical characterization of hybrid multiwall carbon nanotube and fumed silica epoxy nanocomposites. Polym. Plast. Technol. Eng. 2013, 52, 1054–1062. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds CaBG and SrBG are available from the authors. |
















| CaBG | SrBG | |||
|---|---|---|---|---|
| Element | Atomic conc. (%) | Weight conc. (%) | Atomic conc. (%) | Weight conc. (%) |
| O | 64.39 ± 2.87 | 49.58 ± 2.98 | 57.89 ± 2.01 | 38.59 ± 2.10 |
| Si | 31.39 ± 2.75 | 42.33 ± 3.03 | 37.06 ± 1.65 | 43.30 ± 1.11 |
| P | 0.10 ± 0.06 | 0.14 ± 0.09 | 0.13 ± 0.02 | 0.17 ± 0.04 |
| Ca | 4.13 ± 0.19 | 7.94 ± 0.30 | 0 | 0 |
| Sr | 0 | 0 | 4.92 ± 0.38 | 17.94 ± 1.06 |
| Sample | Tm (°C) | DHm (J/g) | Tc (°C) | DHc (J/g) | Xc (%) |
|---|---|---|---|---|---|
| Iba | 142.4, 179.7 | 173.64, 25.90 | − | − | |
| PCL | 58.3 | 73.16 | 34.7 | −57.96 | 54.2 |
| PCL/CaBG | 57.5 | 53.81 | 35.1 | −47.40 | 35.9 |
| PCL/SrBG | 58.5 | 50.83 | 34.6 | −43.04 | 33.9 |
| PCL/CaBG-Iba | 59.2 | 47.60 | 34.8 | −39.81 | 31.7 |
| PCL/SrBG-Iba | 59.4 | 54.75 | 34.1 | −51.06 | 36.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Tzetzis, D.; Bikiaris, D. Composite Membranes of Poly(ε-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules 2019, 24, 3067. https://doi.org/10.3390/molecules24173067
Terzopoulou Z, Baciu D, Gounari E, Steriotis T, Charalambopoulou G, Tzetzis D, Bikiaris D. Composite Membranes of Poly(ε-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules. 2019; 24(17):3067. https://doi.org/10.3390/molecules24173067
Chicago/Turabian StyleTerzopoulou, Zoi, Diana Baciu, Eleni Gounari, Theodore Steriotis, Georgia Charalambopoulou, Dimitrios Tzetzis, and Dimitrios Bikiaris. 2019. "Composite Membranes of Poly(ε-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications" Molecules 24, no. 17: 3067. https://doi.org/10.3390/molecules24173067
APA StyleTerzopoulou, Z., Baciu, D., Gounari, E., Steriotis, T., Charalambopoulou, G., Tzetzis, D., & Bikiaris, D. (2019). Composite Membranes of Poly(ε-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules, 24(17), 3067. https://doi.org/10.3390/molecules24173067