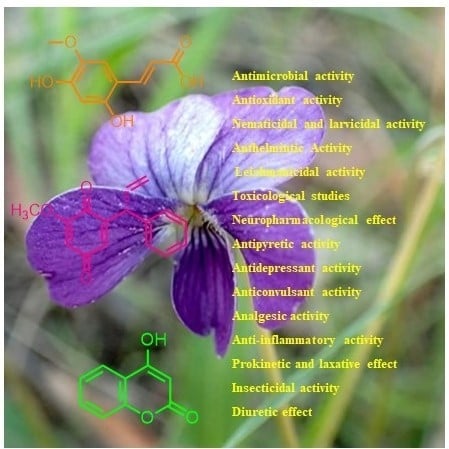
A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits
Abstract
:1. Introduction
2. Methods
3. Morphology, Nutritional, and Traditional Values of Viola betonicifolia
3.1. Microscopy and Physicochemical Characteristics of V. betonicifolia
3.2. Extractive Values of V. betonicifolia with Different Solvents
3.3. Nutritional Values of V. betonicifolia
4. Phytochemistry
5. Pharmaceutical Potential of Viola betonicifolia
5.1. Antimicrobial Activity
5.2. Antioxidant Activity
5.3. Nematicidal and Larvicidal Activity
5.4. Anthelmintic and Leishmanicidal Activity
5.5. Toxicological Studies
5.6. Neuropharmacological Activities
5.7. Antipyretic Activity
5.8. Antidepressant Activity
5.9. Anticonvulsant Activity
5.10. Analgesic Activity
5.11. Anti-Inflammatory Activity
5.12. Prokinetic and Laxative Effects
5.13. Insecticidal Activity
5.14. Diuretic Effect
6. V. betonicifolia in the Cosmetics Industry
7. Volatile Oils Composition of V. betonicifolia
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Introductory Note about Corresponding Author
References
- Kotnis, M.S.; Patel, P.; Menon, S.N.; Sane, R.T. Renoprotective effect of Hemidesmus indicus, a herbal drug used in gentamicin-induced renal toxicity. Nephrology 2004, 9, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rasool, N.; Rizwan, K.; Zubair, M.; Riaz, M.; Zia-Ul-Haq, M.; Rana, U.A.; Nafady, A.; Jaafar, H.Z. Chemical composition and Biological studies of Ficus benjamina. Chem. Cent. J. 2014, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Dahanukar, S.; Kulkarni, R.; Rege, N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000, 32, 81–118. [Google Scholar]
- Harsha, V.; Hebbar, S.; Hegde, G.; Shripathi, V. Ethnomedical knowledge of plants used by Kunabi Tribe of Karnataka in India. Fitoterapia 2002, 73, 281–287. [Google Scholar] [CrossRef]
- Zubair, M.; Hassan, S.; Rizwan, K.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; De Feo, V. Antioxidant potential and oil composition of Callistemon viminalis leaves. Sci. World J. 2013, 2013, 489071. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Nabavi, S.M.; Barreca, D.; Fischer, N.; Efferth, T.J.P.R. Pharmacological and chemical features of Nepeta L. genus: Its importance as a therapeutic agent. Phytother. Res. 2018, 32, 185–198. [Google Scholar]
- Mabberley, D.J. The Plant-Book: A Portable Dictionary of the Vascular Plants; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Perveen, A.; Qaiser, M. Pollen Flora of Pakistan-LXI. Violaceae. Pak. J. Bot. 2009, 41, 1–5. [Google Scholar]
- Mittal, P.; Gupta, V.; Goswami, M.; Thakur, N.; Bansal, P. Phytochemical and pharmacological potential of Viola odorata. Int. J. Pharmacog. 2015, 2, 215–220. [Google Scholar]
- Muhammad, N.; Saeed, M.; Aleem, A.; Khan, H.J.P. Ethnomedicinal, phytochemical and pharmacological profile of genus Viola. Phytopharmacology 2012, 3, 214–226. [Google Scholar]
- Zhu, H.; Qin, S.S.; Zhang, N.; Yang, D.W.; Han, H.R.; Wei, K.H.; Li, M.H.J.C. Chemical constituents and biological activities of plants from the Genus Viola. Chem. Biodivers. 2015, 12, 1777–1808. [Google Scholar] [CrossRef]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V. Phytochemical and biological studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.N.; Zubair, M.; Rizwan, K.; Rasool, N.; Bukhari, I.H.; Akram, S.; Bokhari, T.H.; Shahid, M.; Hameed, M.; Ahmad, V.U. Biological activities of Opuntia Monacantha cladodes. J. Chem. Soc. Pak. 2012, 34, 990–995. [Google Scholar]
- Rizwan, K.; Zubair, M.; Rasool, N.; Mahmood, A.; Ercisli, S.; Zia-Ul-Haq, M.; Dima, L. Compositional studies and antioxidant potential of fruit of Zizyphus oxyphylla edgew. Oxid. Commun. 2016, 39, 1309–1322. [Google Scholar]
- Ashraf, S.N.; Zubair, M.; Rizwan, K.; Tareen, R.B.; Rasool, N.; Zia-Ul-Haq, M.; Ercisli, S. Compositional studies and Biological activities of Perovskia abrotanoides Kar. oils. Biol. Res. 2014, 47, 12. [Google Scholar] [PubMed]
- Zia-Ul-Haq, M.; Stanković, M.S.; Rizwan, K.; Feo, V.D. Grewia asiatica L., a food plant with multiple uses. Molecules 2013, 18, 2663–2682. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Rasool, N.; Bukhari, I.H.; Shahid, M.; Zubair, M.; Rizwan, K.; Rashid, U. In vitro antimicrobial, antioxidant, cytotoxicity and GC-MS analysis of Mazus goodenifolius. Molecules 2012, 17, 14275–14287. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Rasool, N.; Bukhari, I.; Rizwan, K.; Zubair, M.; Javed, F.; Altaf, A.; Qayyum, H. Antioxidant, Antimicrobial Activity and GC-MS analysis of Russelia equsetiformis Essential Oils. Oxid. Commun. 2012, 35, 1027–1037. [Google Scholar]
- Zia-Ul-Haq, M.; Ahmad, S.; Iqbal, S.; Luthria, D.; Amarowicz, R. Antioxidant potential of lentil cultivars commonly consumed in Pakistan. Oxid. Commun. 2011, 34, 820–831. [Google Scholar]
- Zia-Ul-Haq, M.; Ahmed, S.; Rizwani, G.H.; Qayum, M.; Ahmad, S.; Hanif, M. Report: Platelet aggregation inhibition activity of selected legumes of Pakistan. Pak. J. Pharm. Sci. 2012, 25, 863–865. [Google Scholar]
- Zia-Ul-Haq, M.; Raza Shah, M.; Qayum, M.; Ercisli, S. Biological screening of selected flora of Pakistan. Biol. Res. 2012, 45, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Saeed, M.; Khan, H.; Hassan, S.; Gul, F. Evaluation of Viola betonicifolia for its nutrition value. Pak. J. Pharm. Sci 2012, 25, 639–644. [Google Scholar] [PubMed]
- Muhammad, N.; Saeed, M.; Ibrar, M.; Khan, H. Pharmacognostic studies of Viola betonicifolia. Afr. J. Pharm. Pharmacol. 2012, 6, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Qaiser, M.; Omer, S. Flora of Pakistan: No. 166. Violaceae; University of Karachi: Karachi, Pakistan, 1985; p. 6. [Google Scholar]
- Iqbal, I.; Hamayun, M. Studies on the traditional uses of plants of Malam Jabba valley, District Swat, Pakistan. Ethnobot. Leafl. 2005, 2005, 32. [Google Scholar]
- Shinwari, Z.K. Medicinal plants research in Pakistan. J. Med. Plants Res. 2010, 4, 161–176. [Google Scholar]
- Tiwari, J.K.; Ballabha, R.; Tiwari, P. Ethnopaediatrics in Garhwal Himalaya, Uttarakhand, India (Psychomedicine and Medicine). N. Y. Sci. J. 2010, 3, 123–126. [Google Scholar]
- Bhatt, V.; Negi, G. Ethnomedicinal plant resources of Jaunsari tribe of Garhwal Himalaya, Uttaranchal. Indian J. Tradit. Knowl. 2006, 5, 331–335. [Google Scholar]
- Husain, S.Z.; Malik, R.N.; Javaid, M.; Bibi, S. Ethonobotanical properties and uses of medicinal plants of Morgah biodiversity park, Rawalpindi. Pak. J. Bot. 2008, 40, 1897–1911. [Google Scholar]
- Okhale, S.E.; Amanabo, M.O.; Jegede, I.A.; Egharevba, H.O.; Muazzam, I.W.; Kunle, O.F. Phytochemical and pharmacognostic investigation of antidiabetic Scoparia dulcis Linn Scrophulariaceae whole plant grown in Nigeria. Researcher 2010, 2, 7–16. [Google Scholar]
- Berchtold, M.W.; Brinkmeier, H.; Muntener, M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef]
- Drukarch, B.; Holland, H.A.; Velichkov, M.; Geurts, J.J.G.; Voorn, P.; Glas, G.; de Regt, H.W. Thinking about the nerve impulse: A critical analysis of the electricity-centered conception of nerve excitability. Prog. Neurobiol. 2018, 169, 172–185. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M. Biological screening of Viola betonicifolia Smith whole plant. Afr. J. Pharm. Pharmacol. 2011, 5, 2323–2329. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Khan, H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement. Altern. Med. 2012, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; ur Rehman, N.; Khan, H.; Saeed, M.; Gilani, A.-H. Prokinetic and laxative effects of the crude methanolic extract of Viola betonicifolia whole plant in rodents. BMC Complement. Altern. Med. 2013, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Saeed, M.; Adhikari, A.; Khan, K.M.; Khan, H. Isolation of a new bioactive cinnamic acid derivative from the whole plant of Viola betonicifolia. J. Enzym. Inhib. Med. Chem. 2013, 28, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Saeed, M.; Khan, A.; Adhikari, A.; Wadood, A.; Khan, K.M.; De Feo, V. A new urease inhibitor from Viola betonicifolia. Molecules 2014, 19, 16770–16778. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Saeed, M.; Khan, H.; Adhikari, A.; Khan, K.M. Muscle Relaxant and Sedative-Hypnotic Activities of Extract of Viola betonicifolia in Animal Models Supported by Its Isolated Compound, 4-Hydroxy Coumarin. J. Chem. 2013, 2013, 326263. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Qayum, M.; Khan, H. Anti-microbial Screening of Viola betonicifolia. Middle-East J. Scentific Res. 2013, 15, 55–60. [Google Scholar]
- Muhammad, N.; Khan, A.; Saeed, M.; Khan, H.; Khan, S.S.; Shareef, H.; Khan, Z.; Farooq, U.; Zahoor, M. Fixed Oil Composition and Biological Screening of Viola betonicifolia in Different Solvents. J. Chem. Soc. Pak. 2016, 38, 115–121. [Google Scholar]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Ashman, R. Candida albicans: Pathogenesis, immunity and host defence. Res. Immunol. 1998, 149, 281–288. [Google Scholar] [CrossRef]
- Ginter-Hanselmayer, G.; Smolle, J.; Gupta, A. Itraconazole in the treatment of tinea capitis caused by Microsporum canis: Experience in a large cohort. Pediatric Dermatol. 2004, 21, 499–502. [Google Scholar] [CrossRef]
- Feyzabadi, Z.; Ghorbani, F.; Vazani, Y.; Zarshenas, M.M. A critical review on phytochemistry, pharmacology of Viola odorata L. and related multipotential products in traditional Persian medicine. Phytother. Res. 2017, 31, 1669–1675. [Google Scholar] [CrossRef]
- Katoch, M.; Singh, A.; Singh, G.; Wazir, P.; Kumar, R. Phylogeny, antimicrobial, antioxidant and enzyme-producing potential of fungal endophytes found in Viola odorata. Ann. Microbiol. 2017, 67, 529–540. [Google Scholar] [CrossRef]
- Muhammad, Z.-U.-H.; Mansoor, A.; Mussarat, A. Nematicidal activity of selected flora of Pakistan. Pak. J. Bot. 2010, 42, 2119–2123. [Google Scholar]
- Muhammad, N.; Saeed, M.; Khan, H.; Qayum, M.; Badshah, A. Evaluation of Viola betonicifolia for anthelmintic activity. Afr. J. Pharm. Pharmacol. 2012, 6, 698–701. [Google Scholar] [CrossRef]
- Jawale, C.; Kirdak, R.; Dama, L. Larvicidal activity of Cestrum nocturnum on Aedes aegypti. Bangladesh J. Pharmacol. 2010, 5, 39–40. [Google Scholar]
- Muhammad, N.; Saeed, M. Diuretic, insecticidal and leishmanicidal profile of the whole plant of Viola betonicifolia. Afr. J. Pharm. Pharmacol. 2017, 11, 573–576. [Google Scholar]
- Muhammad, N.; Saeed, M.; Khan, H.; Haq, I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J. Nat. Med. 2013, 67, 1–8. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Khan, H.; Raziq, N.; Halimi, S.M.A. Antipyretic and anticonvulsant activity of n-hexane fraction of Viola betonicifolia. Asian Pac. J. Trop. Biomed. 2013, 3, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Saeed, M.; Gilani, S.N. Analgesic and anti-inflammatory profile of n-hexane fraction of viola betonicifolia. Trop. J. Pharm. Res. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Monadi, A.; Rezaie, A. Evaluation of sedative and pre-anesthetic effects of Viola odorata Linn. extract compared with diazepam in rats. Bull. Environ. Pharm. Life Sci. 2013, 2, 125–131. [Google Scholar]
- Sultana, S.; Asif, H.M.; Akhtar, N.; Ahmad, K. Medicinal plants with potential antipyretic activity: A review. Asian Pac. J. Trop. Dis. 2015, 5, S202–S208. [Google Scholar] [CrossRef]
- Khattak, S.G.; Gilani, S.N.; Ikram, M. Antipyretic studies on some indigenous Pakistani medicinal plants. J. Ethnopharmacol. 1985, 14, 45–51. [Google Scholar] [CrossRef]
- Aladeokin, A.C.; Umukoro, S. Psychopharmacological properties of an aqueous extract of Tetracarpidium conophorum Hutch. & Dalziel in mice. J. Nat. Med. 2011, 65, 411–416. [Google Scholar]
- Sayyah, M.; Khodaparast, A.; Yazdi, A.; Sardari, S. Screening of the anticonvulsant activity of some plants from Fabaceae family in experimental seizure models in mice. Daru J. Fac. Pharm. Tehran Univ. Med Sci. 2011, 19, 301. [Google Scholar]
- Ezeja, M.; Omeh, Y.; Ezeigbo, I.; Ekechukwu, A. Evaluation of the analgesic activity of the methanolic stem bark extract of Dialium guineense (Wild). Ann. Med Health Sci. Res. 2011, 1, 55–62. [Google Scholar]
- Hijazi, M.A.; El-Mallah, A.; Aboul-Ela, M.; Ellakany, A. Evaluation of analgesic activity of papaver libanoticum extract in mice: Involvement of opioids receptors. Evid. Based Complement. Altern. Med. 2017, 2017, 8935085. [Google Scholar] [CrossRef]
- Joseph, J.; Bindhu, A.; Aleykutty, N. In vitro and in vivo Antiinflammatory Activity of Clerodendrum paniculatum Linn. Leaves. Indian J. Pharm. Sci. 2013, 75, 376. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Koochek, M.; Pipelzadeh, M.; Mardani, H. The effectiveness of Viola odorata in the prevention and treatment of formalin-induced lung damage in the rat. J. Herbsspices Med. Plants 2003, 10, 95–103. [Google Scholar] [CrossRef]
- Akhlaq, A.; Mehmood, M.H.; Rehman, A.; Ashraf, Z.; Syed, S.; Bawany, S.A.; Gilani, A.H.; Ilyas, M.; Siddiqui, B.S. The prokinetic, laxative, and antidiarrheal effects of Morus nigra: Possible muscarinic, Ca2+ channel blocking, and antimuscarinic mechanisms. Phytother. Res. 2016, 30, 1362–1376. [Google Scholar] [CrossRef]
- DiPalma, J.A.; vB Cleveland, M.; McGowan, J.; Herrera, J.L. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am. J. Gastroenterol. 2007, 102, 1436. [Google Scholar] [CrossRef]
- Vishal, A.; Parveen, K.; Pooja, S.; Kannappan, N.; Kumar, S. Diuretic, laxative and toxicity Studies of Viola odorata aerial parts. Pharm. Online 2009, 1, 739–748. [Google Scholar]
- Tedeschi, P.; Leis, M.; Pezzi, M.; Civolani, S.; Maietti, A.; Brandolini, V. Insecticidal activity and fungitoxicity of plant extracts and components of horseradish (Armoracia rusticana) and garlic (Allium sativum). J. Environ. Sci. Health. Part B 2011, 46, 486–490. [Google Scholar]
- Ríos, N.; Stashenko, E.E.; Duque, J.E. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae). Rev. Bras. Entomol. 2017, 61, 307–311. [Google Scholar] [CrossRef]
- Mostafa, M.; Hossain, H.; Hossain, M.A.; Biswas, P.K.; Haque, M.Z. Insecticidal activity of plant extracts against Tribolium castaneum Herbst. J. Adv. Sci. Res. 2012, 3, 80–84. [Google Scholar]
- Via, S. Cannibalism facilitates the use of a novel environment in the flour beetle, Tribolium castaneum. Heredity 1999, 82, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Hagstrum, D.W.; Subramanyam, B. A review of stored-product entomology information sources. Am. Entomol. 2009, 55, 174–183. [Google Scholar] [CrossRef]
- Sayana, S.B.; Christina, T.M.S.P.; PATIL, P.S. Study of diuretic activity of ethonolic extract of leaves of Cissampelos pareira in rats. Asian J. Pharm. Clin. Res. 2014, 7, 157–159. [Google Scholar]
- Akhtar, M.F. Chemical and Biological Investigations of Medicinal Herbs, Phyla Nodiflora, Ruellia Patula and Ruellia Brittoniana. Ph.D. Thesis, University of Karachi, Karachi, Pakistan, 1993. [Google Scholar]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Cheng, S.-L.; Liu, R.H.; Sheu, J.-N.; Chen, S.-T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J. Biomed. Sci. 2007, 14, 87–105. [Google Scholar] [CrossRef]
- Held, E.; Sveinsdottir, S.; Agner, T. Effect of long-term use of moisturizer on skin hydration, barrier function and susceptibility to irritants. Acta Derm. Venereol. 1999, 79, 49–51. [Google Scholar]



| Morphological Features | Range | Means |
|---|---|---|
| Palisade-ratio | 9–8.25 | 8.62 ± 0.23 |
| Stomata number (upper epidermis) | 12–19 | 16.70 ± 0.31 |
| Epidermal cells (upper epidermis) | 52–73 | 60.70 ± 0. 39 |
| Stomata number (lower epidermis) | 54–62 | 55.23 ± 0.28 |
| Epidermal cells (lower epidermis) | 142–154 | 140.12 ± 0.43 |
| Vein islet number | 7.5–10 | 8.40 ± 0.25 |
| Veinlet termination number | 5–6 | 5.50 ± 0.11 |
| Micronutrients (µg/g) | |||||||
| Plant Parts | Lead (Pb) | Copper (Cu) | Chromium (Cr) | Iron (Fe) | Manganese (Mn) | Nickel (Ni) | Zinc (Zn) |
| Leaves | 6.45 ± 0.21 | 23.90 ± 0.22 | 40 ± 0.15 | 320 ± 0.33 | 14 ± 0.11 | --- | 10 ± 0.15 |
| Petioles | 5.63 ± 0.23 | 15.56 ± 0.11 | 20 ± 0.17 | 340 ± 0.20 | 9 ± 0.22 | 0.50 ± 0.11 | 34 ± 0.26 |
| Root | --- | 42.67 ± 0.26 | 67 ± 0.20 | 295 ± 0.19 | 3 ± 0.02 | --- | 28 ± 0.02 |
| Flower | 1.34 ± 0.26 | 40.89 ± 0.27 | 50 ± 0.10 | 220 ± 0.31 | 27 ± 0.25 | 1.00 ± 0.17 | 36 ± 0.22 |
| Whole Plant | 7.23 ± 0.32 | 80.45 ± 0.22 | 35 ± 0.19 | 245 ± 0.45 | 60 ± 0.22 | 1.20 ± 0.30 | 50 ± 0.25 |
| Macronutrients (µg/g) | |||||||
| Plant Parts | Sodium (Na) | Potassium (K) | Calcium (Ca) | ||||
| Leaves | 156.00 ± 0.12 | 890.00 ± 0.26 | 132.00 ± 0.12 | ||||
| Petioles | 623.00 ± 0.32 | 325.00 ± 0.11 | 256.00 ± 0.24 | ||||
| Root | 214.00 ± 0.38 | 235.00 ± 0.40 | 134.00 ± 0.11 | ||||
| Flower | 124.00 ± 0.44 | 170.00 ± 0.56 | 200.00 ± 0.38 | ||||
| Whole plant | 723.00 ± 0.51 | 191.00 ± 0.122 | 500.00 ± 0.32 | ||||
| Sr # | Pharmacological Activity | Model | Assay | Extract/Fraction/Compounds | Extraction Technique Used | Outcome/Response | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Antibacterial | In vitro E. coli, B. subtilis, S. flexeneri, S. aureus, P. aeruginosa, S. typhi | agar well diffusion method | Methanolic, n-hexane, chloroform, ethyl acetate, n-butanol, aqueous | Maceration and fractionation at room temp. | chloroform fraction showed zone of inhibition against E. coli = 20 mm S. typhi = 17 mm while Ehylacetate fraction showed ZOI against E. coli = 10 mm; S. typhi = 15 mm. n-hexane fraction showed no activity | [39] |
| S. flexeneri, E. coli, B. subtilis, P. aeruginosa, S. typhi, S. aureus | agar well diffusion method | Subfractions F1-F6 | Maceration and fractionation at room temp. | No activity observed | [40] | ||
| Antifungal | T. longifus, C. albicans, F. solani, A. flavus, M. Cani, C. galaberata | Disc diffusion method | Methanolic, n-hexane, chloroform, ethyl acetate, n-butanol, aqueous | Maceration and fractionation at room temp. | % inhibition of methanolic extract against T. longifus 30%, M. Cani; 20%; n-Butanol = nil; Chloroform fraction inhibited M. cani; 20%; F. solani 10%; Ethyl acetate fraction also inhibited growth of different strains, Aqueous fraction inhibited the growth of C. albicans (30%) and M. cani (40%). | [39] | |
| T. longifus, C. albicans, F. solani, A. flavus, M. Cani, C. galaberata | Disc diffusion method | Subfractions F1-F6 | Maceration at room temp. | Potential inhibition effect against all strains was observed | [40] | ||
| 2 | Antioxidant | In vitro | Total phenolic contents | ethylacetate, methanol, chloroform, butanol, Aqueous | Maceration and fractionation at room temp. | chloroform = 62.0 mg/g, methanol = 34.0 mg/g, ethyl acetate = 64.13 mg/g, butanol = 28.32 mg/g, aqueous (6.46 mg/g) | [33] |
| DPPH radical scavenges assay | ethylacetate, methanol, chloroform, butanol, Aqueous, n-hexane | IC50 values of different extracts and fractions, chloroform = 80 μg/m, ethyl acetate = 82 μg/m, methanol = 110 μg/m, n-hexane IC50 = 500 μg/mL, n-butanol = 176 μg/m, aqueous = 496 μg/m | |||||
| Total flavonoid contents | ethylacetate, methanol, chloroform, butanol, Aqueous | Ethyl acetate = 65.36 mg/g, methanol = 39.0 mg/g, chloroform = 63.89 mg/g, n-butanol = 28.0 mg/g, aqueous = 6.86 mg/g | |||||
| 3 | Nematicidal | M. incognita, M. javanica, C. littoralis H. indicus | methanolic chloroform, n-hexane, ethyl acetate, butanol, n-aqueous | Maceration and fractionation at room temp. | Dose and time-dependent mortality effect was observed. After 48 h at 2% conc. M. incognita; ethyl acetate = 77%, chloroform = 71%, methanol = 60% M. javanica; ethyl acetate = 65%, chloroform = 68%, methanolic = 58% C. littoralis; Chloroform = 66%, ethyl acetate = 62 H. indicus; chloroform 49%, ethyl acetate fractions = 57% mortality | [33,47] | |
| Larvecidal | Aedes aegypti | Methanolic extract n-hexane, chloroform, ethyl acetate, n-butanol, aqueous | Maceration and fractionation at room temp. | Chloroform fraction showed LC50 = 13.03 μg/mL, followed by ethyl acetate and methanolic extract 16.00 and 61.30 μg/mL respectively | [33] | ||
| Anthelmintic | Pheretima posthuma (an adult earthworm) | Methanolic extract n-hexane, chloroform, ethyl acetate, n-butanol, aqueous | Maceration and fractionation at room temp. | Time and dose-dependent effect was observed | [47] | ||
| Leishmanicidal | Leishmania major | Methanolic extract n-hexane, chloroform, ethylacetate, n-butanol, aqueous F1-F6 subfractions | Maceration and fractionation at room temp. | IC50 > 100 μg/mL No activity observed No activity observed | [40,49] | ||
| 4 | Toxicological study | Phytotoxic activity | Lemna minor | Methanolic extract n-hexane, chloroform, ethyl acetate, n-butanol, aqueous | Maceration and fractionation at room temp. | The outstanding phytotoxic effects were observed against n-butanol fraction with 83% inhibition while the ethyl acetate fraction showed 73% growth inhibition. Weak effect was observed by other fractions. | [33] |
| F1-F6 subfractions | F1, F3, and F6 showed concentration-dependent phytotoxic effect. All other subfractions showed weak effect. | [40] | |||||
| Cytotoxic activity | Brine shrimp lethality assay | Methanolic extract n-hexane, chloroform, ethylacetate, n-butanol, aqueous | Maceration and fractionation at room temp. | Concentration-dependent effect was observed. Aqueous = LC50 46 μg/mL and chloroform = LC50 56 μg/mL n-hexane = LC50, 60.08 μg/mL | [33] | ||
| F1–F6 subfractions | F5 and F6 (LD50 = 175.4 and 160.7 μL/mL) showed significant cytotoxic effects. F4 did not showed any cytotoxic activity while F1, F2, and F3 showed weak cytotoxic effect. | [40] | |||||
| 5 | Neuropharmacological | In vivo BALB/c mice Anxiolytic activity | Staircase test | methanolic extract n-hexane fraction | Maceration and fractionation at room temp. | Significant Dose-dependent effect observed | [38,50] |
| In vivo BALB/c mice muscle relaxant | Chimney test, Traction test, Rota rod, and Inclined plane | Significant Dose-dependent effect observed | |||||
| In vivo BALB/c mice sleep induction | Hypnotic test and sedative test | n-hexane fraction and methanolic extract notably reduced the latency time and increased the total sleeping duration in dose-dependent manner | |||||
| 6 | Antipyretic | In vivo BALB/c mice | Brewers-induced pyrexia | Methanol extract n-hexane fraction | Maceration and fractionation at room temp. | methaolic extract = 78.23%, n-hexane = 82.50% at dose 300 mg/kg | [34,51] |
| 7 | Antidepressant | In vivo BALB/c mice | Forced swimming test (FST) | n-hexane fraction | Maceration and fractionation at room temp. | No antidepressant effect | [50] |
| locomotor activity by line crossing test | No antidepressant effect | ||||||
| 8 | Analgesic | In vivo BALB/c mice | Acetic acid induced writhing test | Methanolic extract n-hexane fraction | Maceration and fractionation at room temp. | In acetic acid induced analgesia, methanolic extract and n-hexane fraction showed the maximum inhibition 78.9% and 85.2% at 300 mg/kg dose. | [34,52] |
| Tail immersion test | Significant analgesic effect observed | ||||||
| Hot plate test | Significant analgesic effect was observed | ||||||
| 9 | Anticonvulsant | In vivo BALB/c mice | PTZ induced seizures | n-hexane fraction | Maceration and fractionation at room temp. | Dose-dependent effect observed. No mortality observed | [51] |
| strychnine induced convulsions | No activity, all mice died | ||||||
| 10 | Anti-inflammatory | In vivo BALB/c mice | carrageen induced edema Histamine induced edema | Methanolic extract n-hexane fraction | Maceration and fractionation at room temp. | Dose-dependent effect was observed Methanolic extract = 60.9%; n-hexane = 60.8% at 300 mg/kg dose. Methanolic extract showed anti-inflammatory effect in a dose-dependent manner. n-hexane fraction showed no anti-inflammatory effect | [34,52] |
| 11 | Diuretic | In vivo BALB/c mice | Methanolic extract n-hexane fraction | Maceration and fractionation at room temp. | Methnolic extract showed mild diuretic effect but statistically nonsignificant n-hexane fraction showed no activity | [49] | |
| 12 | Prokinetic and laxative effects | In vivo BALB/c mice | spasmogenic effect | Methanolic extract | Maceration and fractionation at room temp. | The crude methanolic extract showed partially atropine-sensitive prokinetic (50 and 100 mg/kg) and laxative (30 and 100 mg/kg) effects | [35] |
| In vitro studies on isolated rabbit jejunum and guinea pig ileum | Methanolic extract showed dose-dependent contractions. The spasmodic effect of methanolic extract was more efficacious in guinea pig ileum than rabbit jejunum preparation. | ||||||
| 13 | Insecticidal | In vitro Tribolium castaneum, Rhyzopertha dominica, and Callosobruchus analis | methanolic extract n-hexane, chloroform, ethylacetate, n-butanol, aqueous | Maceration and fractionation at room temp. | Methanolic extract showed 20 and 40% activity against T. castaneum and C. analis, chloroform fraction showed 40% mortality against T. castaneum. n-hexane fraction showed 40 and 20% mortality against R. dominica and C. analis. Ethyl acetate fraction showed no activity while aqueous fraction showed 40% effects against C. analis | [49] | |
| subfractions (F1–F6) | significant effect observed | [40] |
| Treatment | Dose | Onset of Sleep (Min) | Duration of Sleeping (Min) |
|---|---|---|---|
| Distilled water | 10 mL/kg | 25.12 ± 1.25 | 7.34 ± 2.28 |
| Diazepam | 4 mg/kg | 5.45 ± 0.08 | 56.45 ± 0.00 |
| n-hexane fraction | 0.3 g/kg | 30.45 ± 1.97 | 5.13 ± 0.99 |
| 0.4 g/kg | 13.79 ± 1.98 | 18.08 ± 0.76 | |
| 0.5 g/kg | 9.08 ± 1.01 | 30.03 ± 1.98 | |
| Methanolic extract | 0.3 g/kg | 23.45 ± 0.87 | 8.13 ± 0.97 |
| 0.4 g/kg | 15.78 ± 0.78 | 13.98 ± 1.76 | |
| 0.5 g/kg | 10.98 ± 0.91 | 25.23 ± 1.46 |
| Treatment | Dose | Immobility Time (s) |
|---|---|---|
| Distilled water | 10 mL/kg | 110 ± 0.09 |
| n-hexane fraction | 0.3 mg | 157 ± 1.07 |
| 0.4 mg | 206 ± 1.72 | |
| 0.5 mg | 215 ± 0.93 | |
| Fluoxetine | 15 mg | 30.34 ± 0.00 |
| Sr# | Compound | % Area | ||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | Retention Time | ||
| 1 | 3-Hexanone | - | - | - | 1.48 | - | - | 4.48 |
| 2 | 2-Hexanone | - | - | - | 2.01 | - | - | 4.59 |
| 3 | Heptane | - | - | - | 3.35 | - | - | 5.38 |
| 4 | Dimethyl-1-heptene | - | - | - | 2.68 | - | - | 5.9 |
| 5 | Octane-4-methyl | - | - | - | 1.6 | - | - | 6.5 |
| 6 | 4-Methyldecane | - | - | - | 1.4 | - | - | 10.74 |
| 7 | Dodecane | - | - | - | 1.03 | - | - | 11.95 |
| 8 | Dimethylnonane | - | - | - | 6.11 | - | - | 12.1 |
| 9 | 1-Decanol | - | - | - | 1.7 | - | - | 12.5 |
| 10 | Tricosane | - | - | - | 1.03 | - | - | 13.2 |
| 11 | 3,8-Dimethyl undicane | - | - | - | 1.5 | - | - | 17.11 |
| 12 | Pentadecane | - | - | - | 1.8 | 2.7 | - | 17.4 |
| 13 | 4,6-Dimethyldodecane | - | - | - | 1.99 | - | - | 17.9 |
| 14 | 1-Octadecanol | - | - | - | 1.46 | - | - | 18.09 |
| 15 | Tetradecane | - | - | - | 2.2 | 1.15 | - | 20.15 |
| 16 | Tetracosane | - | - | - | 1.03 | - | - | 21.5 |
| 17 | Docosane | - | - | - | 1.7 | - | - | 22.3 |
| 18 | Cyclohexadecane | - | - | 4.35 | 3.2 | - | - | 22.5 |
| 19 | 2,4-Di-tert-butyl phenol | 4.13 | 3.76 | - | - | 10.6 | - | 22.51 |
| 20 | Tritriacontane | - | - | - | 8.9 | - | - | 22.74 |
| 21 | Octylether | - | - | - | 2.6 | - | - | 22.90 |
| 23 | Tetramethylhexadecane | - | - | - | 9.8 | - | - | 23.27 |
| 24 | Tetradecanol | - | 6.42 | - | 4.3 | - | 24.06 | |
| 25 | Hexadecane | - | 3.15 | 1.89 | 1.5 | 21.74 | - | 24.2 |
| 26 | Nonadecane | - | - | - | 2.78 | - | - | 24.8 |
| 27 | Trimethylpentadecane | - | - | - | 100 | - | - | 25.1 |
| 28 | t-Butyl 7-Methyl-3,5-dioxo-6-octenoate | - | - | - | 14.89 | - | - | 25.27 |
| 29 | Oxalic acid, 6-ethyloct-3-yl isobutylester | - | - | - | 4.61 | - | - | 27.0 |
| 30 | Eicosane, 3-phenyl | - | - | - | 1.75 | - | - | 27.32 |
| 31 | 9-Methylene-fluorene | - | - | - | 1.17 | - | - | 27.32 |
| 32 | n-Pentadecanol | 1.41 | - | - | - | - | - | 27.73 |
| 33 | Octadecane | - | 2.5 | 2.24 | - | 1.43 | - | 27.85 |
| 34 | Methyl laurate | - | 10 | - | - | 28.3 | ||
| 35 | Neophytadiene | 5.54 | 11.52 | 1.11 | - | 2.39 | - | 28.53 |
| 36 | 2-Pentadecanone trimethyl | - | - | - | 10 | - | - | 28.46 |
| 37 | Hexahydrofarnesyl acetone | - | - | 1.09 | - | - | - | 28.64 |
| 38 | 2-Benzenedicarboxylic acid, bis(2methylpropyl) ester (1S*,2R*,5R*,7S*)-,2,-Dimethyl- | - | - | - | - | - | 3.77 | 29.07 |
| 39 | 9-Hexadecanoic acid, methyl ester | 5.96 | - | - | - | - | - | 29.63 |
| 40 | Methylhexadec-9-enoate | - | - | 11.92 | - | - | - | 29.64 |
| 41 | Methyl-palmitoleate | - | 2.88 | - | - | 2.5 | - | 29.92 |
| 42 | Methyl palmitate | - | - | 14.79 | - | 5.17 | - | 29.98 |
| 43 | Methyl 10-methyldodecanate | 10 | - | - | - | - | - | 29.98 |
| 44 | Methyl tridecanoate | - | 1.44 | - | - | - | - | 30.04 |
| 45 | 1 Eicosanol | 1.15 | - | - | - | - | - | 31.06 |
| 46 | Chlorphyrifos | - | 2.79 | - | - | - | - | 31.13 |
| 47 | Eicosane | - | 6.17 | 16.74 | 6.32 | 2.24 | - | 31.16 |
| 48 | 2- Ethyl-1-dodecene | - | - | 3.2 | - | - | - | 31.29 |
| 49 | Methyl myristate | 34.94 | 3.65 | - | - | - | - | 31.58 |
| 50 | Methyl linoleate | 4.7 | - | - | - | - | - | 32.65 |
| 51 | Methyl oleate | 3.62 | 1.63 | - | - | - | 32.73 | |
| 52 | 9,12,15-Octadecatrienoic acid methylester | - | - | 11.37 | - | - | - | 32.77 |
| 53 | Methyl pentadecanoate | 1.34 | - | - | - | - | 33.12 | |
| 54 | 1-Tridecanol | - | 1.1 | - | - | - | 33.51 | |
| 55 | Arachic alcohol | 3.15 | 10.6 | 10.0 | - | - | 34.11 | |
| 56 | n-Heneicocane | - | - | 9.45 | 85.87 | - | - | 34.18 |
| 57 | 7-ethyl-6,8 dioxabicyclo [3.2.1]-oct-3-ene | - | - | - | - | - | 10 | 38.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwan, K.; Khan, S.A.; Ahmad, I.; Rasool, N.; Ibrahim, M.; Zubair, M.; Jaafar, H.Z.; Manea, R. A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits. Molecules 2019, 24, 3138. https://doi.org/10.3390/molecules24173138
Rizwan K, Khan SA, Ahmad I, Rasool N, Ibrahim M, Zubair M, Jaafar HZ, Manea R. A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits. Molecules. 2019; 24(17):3138. https://doi.org/10.3390/molecules24173138
Chicago/Turabian StyleRizwan, Komal, Shakeel Ahmad Khan, Ikram Ahmad, Nasir Rasool, Muhammad Ibrahim, Muhammad Zubair, Hawa ZE Jaafar, and Rosana Manea. 2019. "A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits" Molecules 24, no. 17: 3138. https://doi.org/10.3390/molecules24173138
APA StyleRizwan, K., Khan, S. A., Ahmad, I., Rasool, N., Ibrahim, M., Zubair, M., Jaafar, H. Z., & Manea, R. (2019). A Comprehensive Review on Chemical and Pharmacological Potential of Viola betonicifolia: A Plant with Multiple Benefits. Molecules, 24(17), 3138. https://doi.org/10.3390/molecules24173138