Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging
Abstract
:1. Introduction
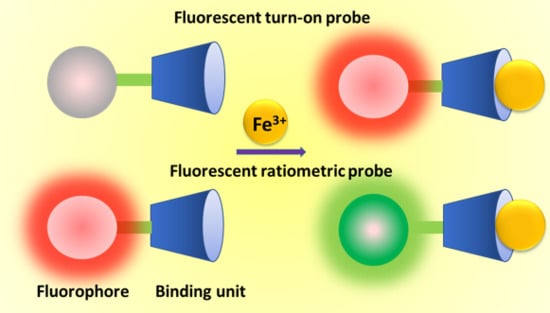
2. Fluorescent Turn-on Probes for Fe(III)


3. Ratiometric Fluorescent Probes for Fe(III)



4. Fluorescent Chemodosimeters for Fe(III)

5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Harigae, H. Iron metabolism and related diseases: An overview. Int. J. Hematol. 2018, 107, 5–6. [Google Scholar] [CrossRef] [PubMed]
- VanderMeulen, H.; Sholzberg, M. Iron deficiency and anemia in patients with inherited bleeding disorders. Transfus. Apher. Sci. 2018, 57, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I. Iron Chelation for Iron Overload in Thalassemia. In (Rditpr), Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal ions in the Clinic; De Gruyter: Boston, MA, USA; Berlin, Germany.
- Kaur, N.; Chopra, S.; Singh, G.; Raj, P.; Bhasin, A.; Sahoo, S.K.; Kuwar, A.; Singh, N. Chemosensors for biogenic amines and biothiols. J. Mater. Chem. B 2018, 6, 4872–4902. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Kim, G.-D.; Choi, H.-J. Optical sensing of anions using C3v-symmetric tripodal receptors. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 30–53. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponi, G.; Callan, J.F. Iron(III) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kuba, A.; Thomas, R.; Kumar, R.; Choi, H.-J.; Sahoo, S.K. An aqueous friendly chemosensor derived from vitamin B6 cofactor for colorimetric sensing of Cu2+ and fluorescent turn-off sensing of Fe3+. Spectrochim. Acta A 2016, 153, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, D.; Moirangthem, A.; Kuba, A.; Thomas, R.; Kumar, R.; Kuwar, A.; Choi, H.-J.; Basu, A. Pyridoxal derived chemosensor for chromogenic sensing of Cu2+ and fluorogenic sensing of Fe3+ in semi-aqueous medium. J. Lumin. 2016, 172, 297–303. [Google Scholar] [CrossRef]
- Na Kim, H.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar] [CrossRef]
- Wei, Y.; Aydin, Z.; Zhang, Y.; Liu, Z.; Guo, M. A turn-on fluorescent sensor for imaging labile Fe3+ in live neuronal cells at subcellular resolution. Chembiochem 2012, 13, 1569–1573. [Google Scholar] [CrossRef]
- Chereddy, N.R.; Thennarasu, S.; Mandal, A.B. Incorporation of triazole into a quinoline-rhodamine conjugate imparts iron(iii) selective complexation permitting detection at nanomolar levels. Dalton Trans. 2012, 41, 11753–11759. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hou, F.; Cheng, J.; Xi, P.; Chen, F.; Bai, D.; Zeng, Z. Selective off–on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Org. Biomol. Chem. 2012, 10, 9634–9638. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-R.; Wu, S.-P. New water-soluble highly selective fluorescent chemosensor for Fe (III) ions and its application to living cell imaging. Sens. Actuators B Chem. 2012, 171, 1110–1116. [Google Scholar] [CrossRef]
- Bordini, J.; Calandreli, I.; Silva, G.O.; Ferreira, K.Q.; Leitão-Mazzi, D.P.; Espreafico, E.M.; Tfouni, E. A rhodamine-B-based turn-on fluorescent sensor for biological iron(III). Inorg. Chem. Commun. 2013, 35, 255–259. [Google Scholar] [CrossRef]
- Saleem, M.; Abdullah, R.; Ali, A.; Park, B.J.; Choi, E.H.; Hong, I.S.; Lee, K.H. Facile synthesis, cytotoxicity and bioimaging of Fe3+ selective fluorescent chemosensor. Bioorg. Med. Chem. 2014, 22, 2045–2051. [Google Scholar] [CrossRef]
- Bao, X.; Shi, J.; Nie, X.; Zhou, B.; Wang, X.; Zhang, L.; Liao, H.; Pang, T. A new Rhodamine B-based ‘on–off’ chemical sensor with high selectivity and sensitivity toward Fe3+ and its imaging in living cells. Bioorg. Med. Chem. 2014, 22, 4826–4835. [Google Scholar] [CrossRef]
- Ji, S.; Meng, X.; Ye, W.; Feng, Y.; Sheng, H.; Cai, Y.; Liu, J.; Zhu, X.; Guo, Q. A rhodamine-based “turn-on” fluorescent probe for Fe3+ in aqueous solution. Dalton Trans. 2014, 43, 1583–1588. [Google Scholar] [CrossRef]
- Sivaraman, G.; Sathiyaraja, V.; Chellappa, D. Turn-on fluorogenic and chromogenic detection of Fe(III) and its application in living cell imaging. J. Lumin- 2014, 145, 480–485. [Google Scholar] [CrossRef]
- Sheng, H.; Meng, X.; Ye, W.; Feng, Y.; Sheng, H.; Wang, X.; Guo, Q. A water-soluble fluorescent probe for Fe(III): Improved selectivity over Cr(III). Sens. Actuators B Chem. 2014, 195, 534–539. [Google Scholar] [CrossRef]
- Meng, W.-F.; Yang, M.-P.; Li, B.; Cheng, Z.; Yang, B.-Q. Fe3+-selective naked-eye ‘off-on’ fluorescent probe: its crystal structure and imaging in living cells. Tetrahedron 2014, 70, 8577–8581. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Z.; She, M.; Sun, W.; Yin, B.; Liu, P.; Zhang, S.; Li, J. Design and synthesis of functionalized rhodamine based probes for specific intracellular fluorescence imaging of Fe3+. Dye. Pigment. 2015, 115, 120–126. [Google Scholar] [CrossRef]
- Bao, X.; Cao, X.; Nie, X.; Xu, Y.; Guo, W.; Zhou, B.; Zhang, L.; Liao, H.; Pang, T. A new selective fluorescent chemical sensor for Fe3+ based on rhodamine B and a 1,4,7,10-tetraoxa-13-azacyclopentadecane conjugate and its imaging in living cells. Sens. Actuators B Chem. 2015, 208, 54–66. [Google Scholar] [CrossRef]
- Yan, F.; Zheng, T.; Shi, D.; Zou, Y.; Wang, Y.; Fu, M.; Chen, L.; Fu, W. Rhodamine-aminopyridine based fluorescent sensors for Fe3+ in water: Synthesis, quantum chemical interpretation and living cell application. Sens. Actuators B Chem. 2015, 215, 598–606. [Google Scholar] [CrossRef]
- Fan, S.; Yang, W.; Hao, J.; Li, H.; Zhao, W.; Zhang, J.; Hu, Y. Cascade OFF–ON–OFF fluorescent probe: Dual detection of Fe3+ ions and thiols. J. Photochem. Photobiol. A Chem. 2016, 328, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Wang, J.; Zhang, D.; Ye, Y.; Zhao, Y. rhodamine-based ‘‘turn-on’’ fluorescent probe for Fe3+ in aqueous solution and its application in bioimaging. Monatsh. Chem. 2016, 147, 311–317. [Google Scholar] [CrossRef]
- Chan, S.; Li, Q.; Tse, H.; Lee, A.W.M.; Mak, N.K.; Lung, H.L.; Chan, W.-H. A rhodamine-based “off–on” fluorescent chemosensor for selective detection of Fe3+ in aqueous media and its application in bioimaging. RSC Adv. 2016, 6, 74389–74393. [Google Scholar] [CrossRef]
- Fan, C.; Huang, X.; Han, L.; Lu, Z.; Wang, Z.; Yi, Y. Novel colorimetric and fluorescent off–on enantiomers with high selectivity for Fe3+ imaging in living cells. Sens. Actuators B Chem. 2016, 224, 592–599. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ru, J.; Yao, X.; Yang, Y.; Li, X.; Tang, X.; Zhang, G.; Liu, W. A naked-eye visible and turn-on fluorescence probe for Fe3+ and its bioimaging application in living cells. Sens. Actuators B Chem. 2016, 237, 501–508. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Liu, Q.; Wang, W. A novel colorimetric and OFF–ON fluorescent chemosensor based on fluorescein derivative for the detection of Fe3+ in aqueous solution and living cells. Tetrahedron Lett. 2016, 57, 1852–1855. [Google Scholar] [CrossRef]
- Wang, K.P.; Chen, J.P.; Zhang, S.J.; Lei, Y.; Zhong, H.; Chen, S.; Zhou, X.H.; Hu, Z.Q. Thiophene-based rhodamine as selective fluorescence probe for Fe(III) and Al(III) in living cells. Anal. Bioanal. Chem. 2017, 409, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumari, C.; Sain, D.; Hira, S.K.; Manna, P.P.; Dey, S. Synthesis of Rhodamine-Based Chemosensor for Fe3+ Selective Detection with off–on Mechanism and its Biological Application in DL-Tumor Cells. ChemistrySelect 2017, 2, 2969–2974. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, H.; Liu, Q.; Wang, W. A novel turn-on colorimetric and fluorescent sensor for Fe3+ and its application in living cells. J. Photochem. Photobiol. A Chem. 2017, 332, 351–356. [Google Scholar]
- Alam, R.; Bhowmick, R.; Islam, A.S.M.; Chaudhuri, K.; Ali, M. A rhodamine based fluorescent trivalent sensor (Fe3+, Al3+, Cr3+) with potential applications for live cell imaging and combinational logic circuits and memory devices. New J. Chem. 2017, 41, 8359–8369. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, H.Q.; Wu, W.N.; Zhao, X.L.; Yang, Y.; Xu, Z.Q.; Xu, Z.H.; Jia, L. Rhodamine-2-thioxoquinazolin-4-one conjugate: A highly sensitive and selective chemosensor for Fe3+ ions and crystal structures of its Ag(I) and Hg(II) complexes. Sens. Actuators B Chem. 2017, 239, 60–68. [Google Scholar] [CrossRef]
- Wang, K.P.; Lei, Y.; Zhang, S.J.; Zheng, W.J.; Chen, J.P.; Chen, S.; Zhang, Q.; Zhang, Y.B.; Hu, Z.Q. Fluorescent probe for Fe(III) with high selectivity and its application in living cells. Sens. Actuators B Chem. 2017, 252, 1140–1145. [Google Scholar] [CrossRef]
- Chen, H.; Bao, X.; Shu, H.; Zhou, B.; Ye, R.; Zhu, J. Synthesis and evaluation of a novel rhodamine B-based ‘off-on’ fluorescent chemosensor for the selective determination of Fe3+ ions. Sens. Actuators B Chem. 2017, 242, 921–931. [Google Scholar] [CrossRef]
- Jin, X.; Wang, S.; Yin, W.; Xu, T.; Jiang, Y.; Liao, Q.; Xia, X.; Liu, J. A highly sensitive and selective fluorescence chemosensor for Fe3+ based on rhodamine and its application in vivo imaging. Sens. Actuators B Chem. 2017, 247, 461–468. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, X.; Hua, Q.; Lei, W.; Hao, Q.; Zhou, B.; Su, C.; Bao, X. Synthesis and evaluation of a new furfuran-based rhodamine B fluorescent chemosensor for selective detection of Fe3+ and its application in living-cell imaging. Sens. Actuators B Chem. 2017, 253, 292–301. [Google Scholar] [CrossRef]
- Ozdemir, M.; Zhang, Y.; Guo, M. A highly selective “off-on” fluorescent sensor for subcellular visualization of labile iron(III) in living cells. Inorg. Chem. Commun. 2018, 90, 73–77. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Zhi, W.; Wang, Y.; Han, J.; Cao, Z.; Ni, L.; Li, H.; Jing, J. A water-soluble Fe3+ selective fluorescent turn-on chemosensor: Preparation, theoretical study and its optical vitro imaging. J. Lumin. 2018, 196, 379–386. [Google Scholar] [CrossRef]
- Wang, J.; Long, L.; Xiao, G.; Fang, F. Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water. Open Chem. 2018, 16, 1268–1274. [Google Scholar] [CrossRef] [Green Version]
- Murugan, A.S.; Vidhyalakshmi, N.; Ramesh, U.; Annaraj, J. In vivo bio-imaging studies of highly selective, sensitive rhodamine based fluorescent chemosensor for the detection of Cu2+/Fe3+ ions. Sens. Actuators B. Chem. 2018, 274, 22–29. [Google Scholar] [CrossRef]
- Wang, Y.; Song, F.; Zhu, J.; Zhang, Y.; Du, L.; Kan, C. Highly selective fluorescent probe based on a rhodamine B and furan-2-carbonyl chloride conjugate for detection of Fe3+ in cells. Tetrahedron Lett. 2018, 59, 3756–3762. [Google Scholar] [CrossRef]
- Song, F.; Yang, C.; Liu, H.; Gao, Z.; Zhu, J.; Bao, X.; Kan, C. Dual-binding pyridine and rhodamine B conjugate derivatives as fluorescent chemosensors for ferric ions in aqueous media and living cells. Analyst 2019, 144, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, F.; Bai, Y.; Ding, X.; Sun, W. A Highly Selective ‘Turn-on’ Fluorescent Probe for Detection of Fe3+ in Cells. J. Fluores. 2019, 29, 425–434. [Google Scholar] [CrossRef]
- Gao, Z.; Kan, C.; Liu, H.; Zhu, J.; Bao, X. A highly sensitive and selective fluorescent probe for Fe3+ containing two rhodamine B and thiocarbonyl moieties and its application to live cell imaging. Tetrahedron 2019, 75, 1223–1230. [Google Scholar] [CrossRef]
- Sui, B.; Tang, S.; Liu, T.; Kim, B.; Belfield, K.D. Novel BODIPY-Based Fluorescence Turn-on Sensor for Fe3+ and Its Bioimaging Application in Living Cells. ACS Appl. Mater. Interfaces 2014, 6, 18408–18412. [Google Scholar] [CrossRef]
- Pathak, R.K.; Dessingou, J.; Hinge, V.K.; Thawari, A.G.; Basu, S.K.; Rao, C.P. Quinoline Driven Fluorescence Turn On 1,3-Bis-calix [4]arene Conjugate-Based Receptor to Discriminate Fe3+ from Fe2+. Anal. Chem. 2013, 85, 3707–3714. [Google Scholar] [CrossRef]
- Nandre, J.; Patil, S.; Patil, V.; Yu, F.; Chen, L.; Sahoo, S.; Prior, T.; Redshaw, C.; Mahulikar, P.; Patil, U. A novel fluorescent “turn-on” chemosensor for nanomolar detection of Fe(III) from aqueous solution and its application in living cells imaging. Biosens. Bioelectron. 2014, 61, 612–617. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Li, Y.; Wu, F.; Mao, J.; Yuan, Y.; Cui, Y.; Sun, G.; Zhang, G. A differentially selective probe based on diketopyrrolopyrrole with fluorescence turn-on response to Fe3+, and dual-mode turn-on and ratiometric response to Au3+ and its application in living cell imaging. Biosens. Bioelectron. 2016, 80, 288–293. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, C.; Chen, H.; Hu, M.; He, W.; Guo, Z. A turn-on fluorescent Fe3+ sensor derived from an anthracene-bearing bisdiene macrocycle and its intracellular imaging application. Chem. Commun. 2014, 50, 4631–4634. [Google Scholar] [CrossRef] [PubMed]
- Pandith, A.; Choi, J.H.; Jung, O.S.; Kim, H.S. A simple and robust PET-based anthracene-appended O-N-O chelate for sequential recognition of Fe3+/CN– ions in aqueous media and its multimodal applications. Inorg. Chim. Acta 2018, 482, 669–680. [Google Scholar] [CrossRef]
- Lim, B.; Baek, B.; Jang, K.; Lee, N.K.; Lee, J.H.; Lee, Y.; Kim, J.; Park, J.; Kim, S.; Kang, N.W.; et al. Novel turn-on fluorescent biosensors for selective detection of cellular Fe3+ in lysosomes: Thiophene as a selectivity-tuning handle for Fe3+ sensors. Dye. Pigment. 2019, 169, 51–59. [Google Scholar] [CrossRef]
- Simon, T.; Shellaiah, M.; Srinivasadesikan, V.; Lin, C.C.; Ko, F.H.; Sun, K.W.; Lin, M.C. A simple pyrene based AIEE active Schiff base probe for selective naked eye and fluorescence off–on detection of trivalent cations with live cell application. Sens. Actuators B Chem. 2016, 231, 18–29. [Google Scholar] [CrossRef]
- Chung, P.K.; Liu, S.R.; Wang, H.F.; Wu, S.P. A Pyrene-based Highly Selective Turn-on Fluorescent Chemosensor for Iron(III) Ions and its Application in Living Cell Imaging. J. Fluoresc. 2013, 23, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Huang, T.; Liu, Q.; Xu, H.; Zhuang, Y.; Li, J.; Hu, J.; Wang, A.; Xu, K. Design and synthesis of a highly sensitive “Turn-On” fluorescent organic nanoprobe for iron(iii) detection and imaging. J. Mater. Chem. C 2014, 2, 9077–9082. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Gupta, R.C.; Ali, R.; Razi, S.S.; Hira, S.K.; Manna, P.P.; Misra, A. Smart PET based organic scaffold exhibiting bright “Turn–On” green fluorescence to detect Fe3+ ion: Live cell imaging and logic implication. J. Photochem. Photobiol. A Chem. 2018, 358, 157–166. [Google Scholar] [CrossRef]
- Kundu, B.K.; Singh, R.; Tiwari, R.; Nayak, D.; Mukhopadhyay, S. Amide probe as selective Al3+ and Fe3+ sensor inside the HeLa, and A549 cell lines: Pictet-Spengler reaction for rapid detection of tryptophan amino acid. New J. Chem. 2019, 43, 4867–4877. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, J.; Park, S.; Jung, J.; Kim, D. A Schiff Base Fluorescence Enhancement Probe for Fe(III) and Its Sensing Applications in Cancer Cells. Sensors 2019, 19, 2500. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, T.; Kaleeswaran, P.; Pitchumani, K. A highly selective, sensitive and “turn-on” fluorescent sensor for the paramagnetic Fe3+ ion. Sens. Actuators B Chem. 2016, 230, 199–205. [Google Scholar] [CrossRef]
- Erdemir, S.; Kocyigit, O. Anthracene excimer-based “turn on” fluorescent sensor for Cr3+ and Fe3+ ions: Its application to living cells. Talanta 2016, 58, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, N.R.; Thennarasu, S.; Mandal, A.B. A highly selective and efficient single molecular FRET based sensor for ratiometric detection of Fe3+ ions. Analyst 2013, 138, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, N.R.; Nagaraju, P.; Raju, M.N.; Krishnaswamy, V.R.; Korrapati, P.S.; Bangal, P.R.; Rao, V.J. A novel FRET ‘off–on’ fluorescent probe for the selective detection of Fe3+, Al3+ and Cr3+ ions: Its ultrafast energy transfer kinetics and application in live cell imaging. Biosens. Bioelectron. 2015, 68, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Lohar, S.; Banerjee, A.; Sahana, A.; Banik, A.; Mukhopadhyay, S.K.; Das, D. A rhodamine–naphthalene conjugate as a FRET based sensor for Cr3+ and Fe3+ with cell staining application. Anal. Methods 2013, 5, 442–445. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Li, J.-B.; Liang, L.-H.; Lu, D.-Q.; Zhang, J.; Mao, G.-J.; Zhou, L.-Y.; Zhang, X.-B.; Tan, W.; Shen, G.-L.; et al. A rhodamine-appended water-soluble conjugated polymer: an efficient ratiometric fluorescence sensing platform for intracellular metal-ion probing. Chem. Commun. 2014, 50, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.D.; Gong, W.T.; Ye, Z.Q.; Lin, Y.; Ning, G.L. FRET-based ratiometric fluorescent probes for selective Fe3+ sensing and their applications in mitochondria. Dalton Trans. 2013, 42, 10093–10096. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Guo, F.; Xia, R.; Wang, Y.; Yao, D.; Yang, G.; Xie, L. A rhodamine–dansyl conjugate as a FRET based sensor for Fe3+ in the red spectral region. J. Lumin. 2014, 145, 849–854. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Cheng, J.; Song, J.; Zhao, Y.; Ye, Y. Efficient FRET-based fluorescent ratiometric chemosensors for Fe3+ and its application in living cells. J. Lumin. 2015, 157, 143–148. [Google Scholar] [CrossRef]
- Das, S.; Aich, K.; Goswami, S.; Quah, C.K.; Fun, H.K. FRET-based fluorescence ratiometric and colorimetric sensor to discriminate Fe3+ from Fe2+. New J. Chem. 2016, 40, 6414–6420. [Google Scholar] [CrossRef]
- Qin, J.C.; Yang, Z.Y.; Wang, G.Q.; Li, C.R. FRET-based rhodamine–coumarin conjugate as a Fe3+ selective ratiometric fluorescent sensor in aqueous media. Tetrahedron Lett. 2015, 56, 5024–5029. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Huang, X.; Ding, P.; Wang, Z.; Zhao, Y.; Ye, Y. A fluorescence ratiometric chemosensor for Fe3+ based on TBET and its application in living cells. Talanta 2014, 128, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Sarkar, S.; Chattopadhyay, B.; Moirangthem, A.; Basu, A.; Dhara, K.; Chattopadhyay, P. A ratiometric fluorescent chemosensor for iron: discrimination of Fe2+ and Fe3+ and living cell application. Analyst 2012, 137, 3335–3342. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Tarafdar, D. Piperazine-based new sensor: selective ratiometric sensing of Fe31, logic gate construction and cell imaging. Supramol. Chem. 2015, 27, 224–232. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Xue, W.; Wu, A. A benzimidazole-based ratiometric fluorescent sensor for Cr3+ and Fe3+ in aqueous solution. Dye. Pigment. 2013, 97, 475–480. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.-Y.; Zheng, M.-H.; Zhao, X.-L.; Xie, Y.-Z.; Jin, J.-Y. 2-Pyridylthiazole derivative as ICT-based ratiometric fluorescent sensor for Fe(III). Tetrahedron Lett. 2016, 57, 2399–2402. [Google Scholar] [CrossRef]
- Kuwar, A.; Patil, R.; Singh, A.; Sahoo, S.K.; Marek, J.; Singh, N. A two-in-one dual channel chemosensor for Fe3+ and Cu2+ with nanomolar detection mimicking the IMPLICATION logic gate. J. Mater. Chem. C 2015, 3, 453–460. [Google Scholar] [CrossRef]
- Du, J.; Hu, M.; Fan, J.; Peng, X. Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511. [Google Scholar] [CrossRef]
- Wang, R.; Yu, F.; Liu, P.; Chen, L. A turn-on fluorescent probe based on hydroxylamine oxidation for detecting ferric ion selectively in living cells. Chem. Commun. 2012, 48, 5310–5312. [Google Scholar] [CrossRef]
- Long, L.; Zhou, L.; Wang, L.; Meng, S.; Gong, A.; Zhang, C. A ratiometric fluorescent probe for iron(III) and its application fordetection of iron(III) in human blood serum. Anal. Chim. Acta 2014, 812, 145–151. [Google Scholar] [CrossRef]
- Upadhyay, Y.; Anand, T.; Babu, L.T.; Paira, P.; SK, A.K.; Kumar, R.; Sahoo, S.K. Combined use of spectrophotometer and smartphone for the optical detection of Fe3+ using a vitamin B6 cofactor conjugated pyrene derivative and its application in live cells imaging. J. Photochem. Photobiol. A Chem. 2018, 361, 34–40. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Bhorge, Y.R.; Pape, A.J.; Divate, R.D.; Chung, Y.-C.; Yen, Y.-P. A New Schiff Base Chemodosimeter for Fluorescent Imaging of Ferric Ions in Living Cells. J. Fluoresc. 2015, 25, 1331–1337. [Google Scholar] [CrossRef]
- Tsai, H.-T.; Bhorge, Y.R.; Pape, A.J.; Janaki, S.N.; Yen, Y.-P. A Selective Colorimetric and Fluorescent Chemodosimeter for Fe(III) Ion Based on Hydrolysis of Schiff Base. J. Chin. Chem. Soc. 2015, 62, 316–320. [Google Scholar] [CrossRef]
- Chiang, Y.-K.; Bhorge, Y.R.; Divate, R.D.; Chung, Y.-C.; Yen, Y.-P. A New Schiff Base Chemodosimeter: Selective Colorimetric and Fluorescent Detection of Fe (III) Ion. J. Fluoresc. 2016, 26, 1699–1708. [Google Scholar] [CrossRef]
- Venkateswarulu, M.; Mukherjee, T.; Mukherjee, S.; Koner, R.R. Turn-on trivalent cation selective chemodosimetric probe to image native cellular iron pools. Dalton Trans. 2014, 43, 5269. [Google Scholar] [CrossRef]












| Probes | Rh/FL | Rh/FL-Y | Medium | Ex./Em. λ (nm) | LOD | Cells Imaging | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | RhB |  | CH3CN/Tris-HCl buffer (pH 7.3, 2:1, v/v) | 510/580 | 0.1 μM | Human SH-SY5Y | [11] |
| 2 | Rh6G |  | Tris HCl-CH3CN (1:1, v/v, pH 7.4) | 510/552 | 50 nM | Fibroblast | [12] |
| 3 | Rh6G |  | EtOH-H2O (3:7, v/v) | 505/559 | - | EJ (cysticcancer) | [13] |
| 4 | RhB |  | H2O-MeOH (v/v = 9:1, Tris-HCl, pH = 7.4) | 564/588 | 2.2 μM | HeLa | [14] |
| 5 | RhB |  | MeOH-H2O (1:1, v/v, Tris-HCl, pH = 7) | 520/582 | - | B16-F10 murine melanoma cells | [15] |
| 6 | RhB |  | H2O-MeOH (60:40, v/v) at pH 7 | -/599 | 3 μM | L-929 | [16] |
| 7 | RhB |  | MeOH/H2O (3:2, v/v, pH 7.10, HEPES buffer) | 558/581 | 0.031 μM | Human liver cells (L-02) | [17] |
| 8 | RhB |  | MeOH/H2O (1/99) buffer (pH 7, 20 mM HEPES, 50 mM NaNO3) | 530/586 | - | 7402 cells | [18] |
| 9 | Rh6G |  | DMSO/H2O (2:8, 50 mM PBS buffered, pH = 7.4) | 480/553 | 66 nM | Candida albicans cells | [19] |
| 10 | Rh6G |  | DMSO/H2O (2:8, 50 mM PBS buffered, pH = 7.4) | 480/553 | 44.5 nM | Candida albicans cells | [19] |
| 11 | RhB |  | MeOH/H2O (1/99) buffer (pH 7, 20 mM HEPES, 50 mM NaNO3) | 530/586 | - | 293FT cells | [20] |
| 12 | RhB |  | MeOH/H2O (1:1, v/v) | 555/584 | 0.32 μM | HepG2 (liver cells) | [21] |
| 13 | RhB |  | EtOH-H2O (5:5, v/v, PBS, pH = 7.4) | 530/583 | 0.9 μM | L929 cells | [22] |
| 14 | RhB |  | EtOH-H2O (5:5, v/v, PBS pH = 7.4) | 530/583 | 5 μM | L929 cells | [22] |
| 15 | RhB |  | MeOH/H2O (1:1, v/v, pH = 7.4) | 558/581 | 0.396 μM | Neuronal cell line PC12 cells | [23] |
| 16 | RhB |  | H2O | 520/582 | 41 nM | HL-7702 cells | [24] |
| 17 | RhB |  | EtOH-H2O (4/1, v/v, pH 7) | 455/581 | - | Hela | [25] |
| 18 | RhB |  | CH3OH, H2O (3/7, Tris-HCl buffer, pH = 7.40) | 535/579 | 3.49 μM | MGC-803 cells | [26] |
| 19 | RhB |  | (1: 1 = H2O: CH3CN, v/v) | 540/580 | 0.26 μM | NPC-C666 cells | [27] |
| 20 | RhB |  | HEPES buffer, pH = 7, THF/H2O 3/7, v/v | 510/585 | 183 nM | HeLa | [28] |
| 21 | RhB |  | MeOH/H2O (1:1, v/v) | 500/560 | 57 nM | A549 cells | [29] |
| 22 | FL |  | DMSO/H2O (3:7/v:v) HEPES buffered pH 7.2 | 393/515 | 7.4 nM | Hep G2 cells | [30] |
| 23 | Rh6G |  | C2H5OH/H2O (1:1, v/v) | 510/555 | 6 μM | HeLa | [31] |
| 24 | RhB |  | EtOH-H2O, 6:4/v:v, Ph = 7.2, HEPES buffer | 450/582 | 17 nM | DL tumor cells | [32] |
| 25 | RhB |  | EtOH/H2O (9:1, v/v; HEPES buffer, pH = 7.2) | 510/588 | 0.768 μM | HeLa | [33] |
| 26 | Rh6G |  | MeOH/H2O (1:1, v/v, pH 7.2, 40 mM HEPES buffer) | 510/555 | 0.29 μM | HepG2 | [34] |
| 27 | Rh6G |  | CH3CN/HEPES (10 mM, pH = 7.40, 8/2, v/v) | 495/555 | 4.11 μM | U251 cells | [35] |
| 28 | RhB |  | EtOH-H2O (1:4) | 540/580 | 0.13 μM | HeLa | [36] |
| 29 | RhB |  | Tris-HCl (1 mM, pH = 7.4) | 562/584 | 11.6 nM | HepG2 cells | [37] |
| 30 | RhB |  | EtOH/Tris-HCl buffer (v/v, 1/9, pH = 7) | 560/582 | 42 nM | HeLa | [38] |
| 31 | RhB |  | EtOH/H2O (1:1,v/v) | 560/582 | 0.025 μM | HeLa | [39] |
| 32 | RhB |  | CH3CN/Tris-HCl buffer (10 mM, pH 7.3, v/v, 2:1) | 535/583 | - | Bovine aortic endothelial cells | [40] |
| 33 | RhB |  | HEPES buffer (1 mM, pH 7) | 560/596 | 92 nM | Human colon cancer cells SW480 | [41] |
| 34 | Rh6G |  | H2O (containing 1% DMSO as a co-solvent) | 500/556 | 1.2 μM | HeLa | [42] |
| 35 | Rh6G |  | H2O | 510/572 | 33 nM | Zebra fish embryos | [43] |
| 36 | RhB |  | MeOH/H2O (1:1, v/v, pH 7.36, HEPES buffer) | 560/582 | 0.437 μM | MCF-7 cells | [44] |
| 37 | RhB |  | EtOH/H2O (3:1, v/v, HEPES, pH = 7.33) | 560/582 | 0.067 μM | MCF-7 cells | [45] |
| 38 | RhB |  | EtOH/H2O (3:1, v/v, HEPES, pH = 7.33) | 560/582 | 0.345 μM | MCF-7 cells | [45] |
| 39 | RhB |  | EtOH/H2O (v/v, 3/2) | 550/580 | 11.8 nM | SGC7901 (stomach cells) | [46] |
| 40 | RhB |  | EtOH/H2O (2:1, v/v, HEPES buffer, pH 7.20) | 558/582 | 0.205 μM | HeLa | [47] |
| Probes (L) | Medium | Ex. λ (nm) | Em. λ (nm) | Fe3+:L | LOD | Ref. |
|---|---|---|---|---|---|---|
| 41 | H2O-CH3CN (9:1, v/v) | 480 | 512 | 1:1 | 0.13 μM | [48] |
| 42 | CH3CN | 310 | 418 | 1:1 | 0.334 μM | [49] |
| 43 | Aqueous CH3CN | 314 | 554 | 1:1 | 0.74 nM | [50] |
| 44 | EtOH/0.01 M PBS buffer (v/v, 1:1, pH 7.4) | 465 | 578 | 2:1 | 8 nM | [51] |
| 45 | MeOH-H2O (1:1, v/v, TRIS-HCl buffer, pH = 7.2) | 373 | 398,421,447 | 2:1 | 0.58 μM | [52] |
| 46 | CH3CN:H2O (3:7, v/v) at pH 7 | 376 | 406,429,456 | 2:1 | 0.1 pM | [53] |
| 47 | H2O | 420 | 458 | 1:2 | 1 μM | [54] |
| 48 | CH3CN | 395 | 500 | 1:2 | 0.106 μM | [55] |
| 49 | CH3CN-acetone (v/v = 99:1) | 382 | 501 | 1:1 | - | [56] |
| 50 | CH3CN:H2O (3:7, v/v) | 403 | 524 | - | 0.35 nM | [57] |
| 51 | THF:H2O (6:4, v/v) | 408 | 528 | 1:1 | 0.373 μM | [58] |
| 52 | H2O | 332 | 430 | 1:1 | 0.235 μM | [59] |
| 53 | H2O:EtOH (6:4, v/v) | 397 | 550 | 1:1 | 0.8 ppb | [60] |
| 54 | CH3CN:H2O (1:1, v/v) | 380 | 482 | 1:1 | 0.89 nM | [61] |
| 55 | CH3CN | 380 | 516 | 1:2 | 0.45 μM | [62] |
| Probes (L) | Medium | Ex.λ (nm) | Em.λ (nm) | Fe3+: L | LOD | Ref. |
|---|---|---|---|---|---|---|
| 56 | Tris HCl-CH3CN (1:1, v/v, pH 7.4) | 420 | 532, 580 | 1:1 | 50 nM | [64] |
| 57 | Tris HCl-CH3CN (1:1, v/v, pH 7.4) | 400 | 532, 583 | 1:1 | 0.03 μM | [65] |
| 58 | EtOH-H2O (2:1, v/v, HEPES buffer, pH 7) | 330 | 455, 585 | 1:1 | 540 nM | [66] |
| 59 | Tris-HCl, Ph = 7.2 | 400 | 442, 538 | 1:1 | 300 nM | [67] |
| 60 | EtOH-H2O (4:1) | 371 | 431, 594 | 1:1 | 6.93 μM | [68] |
| 61 | CH3CN-Tris buffer (9:1, v/v, pH 7.05) | 380 | 515, 605 | 1:1 | 0.64 μM | [69] |
| 62 | EtOH | 420 | 520, 577 | 1:1 | 0.418 μM | [70] |
| 63 | CH3OH/H2O (2/3, v/v, pH = 7.2) | 370 | 470, 558 | 1:1 | 53.9 nM | [71] |
| 64 | EtOH-H2O (9:1, v/v, Tris-HCl, pH = 7.4) | 450 | 475, 550 | 1:1 | 4.05 μM | [72] |
| 65 | CH3OH-H2O (4:6, v/v) | 420 | 535, 585 | 1:1 | 0.105 μM | [73] |
| 66 | CH3CN-HEPES buffer (1/4, v/v, pH 7.4) | 365 | 412, 445 | 1:1 | 3.5 μM | [74] |
| 67 | CHCl3-MeOH (1:1, v/v) | 300 | 400, 480 | 1:1 | 1.17 μM | [75] |
| 68 | DMSO/H2O (1:99, v/v v/v) | 320 | 443, 380 | 1:1 | 2 μM | [76] |
| 69 | CH3CN/Tris buffer = 9:1, v/v, pH = 7.4 | 380 | 431, 517 | 1:2 | 4.47 μM | [77] |
| 70 | Aqueous CH3CN | 420 | 504, 526 | 1:1 | 10 nM | [78] |
| Probes (L) | Medium | Ex. λ (nm) | Em. λ (nm) | LOD | Ref. |
|---|---|---|---|---|---|
| 71 | HEPES aqueous buffer (pH 7, 40 mM) | 585 | 615 | - | [80] |
| 72 | Potassium phosphate buffer/acetone (1:4, v/v, pH = 7) | 360 | 522 | 0.12 μM | [81] |
| 73 | Aqueous DMSO | 325 | 441 | 4.3 μM | [82] |
| 74 | DMSO/H2O (v/v =70:30) | 396 | 440 | 1.37 μM | [83] |
| 75 | DMSO/H2O (v/v = 9/1, HEPES buffer, pH = 7.4) | 390 | 440 | 75.7 nM | [84] |
| 76 | MeOH/H2O (9/1, v/v) | 388 | 430 | 0.118 μM | [85] |
| 77 | THF-H2O (8:2) | 330 | 430 | 0.38 nM | [86] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahoo, S.K.; Crisponi, G. Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging. Molecules 2019, 24, 3267. https://doi.org/10.3390/molecules24183267
Sahoo SK, Crisponi G. Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging. Molecules. 2019; 24(18):3267. https://doi.org/10.3390/molecules24183267
Chicago/Turabian StyleSahoo, Suban K., and Guido Crisponi. 2019. "Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging" Molecules 24, no. 18: 3267. https://doi.org/10.3390/molecules24183267
APA StyleSahoo, S. K., & Crisponi, G. (2019). Recent Advances on Iron(III) Selective Fluorescent Probes with Possible Applications in Bioimaging. Molecules, 24(18), 3267. https://doi.org/10.3390/molecules24183267