After In Vitro Digestion, Jackfruit Flake Affords Protection against Acrylamide-Induced Oxidative Damage
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic Content and Total Antioxidant Capacity
2.2. Qualitative Identification of Compounds
2.3. Effect of Samples before and after Digestion on AA-Induced Cytotoxicity
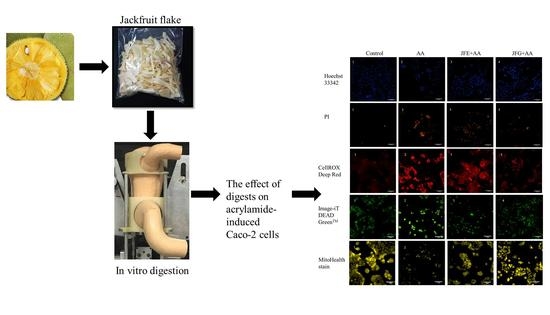
2.4. The Effects of Samples before and after Digestion in AA-Induced ROS Levels
2.5. Samples before and after Digestion Attenuated AA-Induced Oxidative Damage to Mitochondrial Membrane Potential
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.3. Extraction of the Flake
4.4. Simulated Gastrointestinal Digestion of Jackfruit Flake
4.5. Characterization of Total Polyphenols and Antioxidant Capacity
4.5.1. Determination of Total Phenolic Content
4.5.2. Determination of Total Antioxidant Capacity
4.5.3. Identification of Phenolic Compounds by HPLC-MS
4.6. Cell Culture
4.7. Protective Effect against Acrylamide-induced Caco-2 Cell Oxidative Damage
4.7.1. Cell Viability Assay
4.7.2. Detection of Apoptosis
4.7.3. Detection of Intracellular ROS Levels
4.7.4. Detection of Mitochondrial Membrane Potential and Mitochondrial Membrane Permeability
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Zeng, T.; Zhu, Y.B.; Yu, S.F.; Wang, Q.S.; Zhang, L.P.; Guo, X.; Xie, K.Q. Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem. Res. 2008, 33, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Ferreres, F.; Gil-Izquierdo, A.; Oliveira, A.P.; Valentao, P.; Andrade, P.B. Accumulation of primary and secondary metabolites in edible jackfruit seed tissues and scavenging of reactive nitrogen species. Food Chem. 2017, 233, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit): A review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Xie, X.; Wang, H.; Wang, H.; Wang, Z.X.; Sha, X.M.; Lu, Y. Jackfruit (Artocarpus heterophyllus Lam.) peel: A better source of antioxidants and a-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem. 2017, 234, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Montanez, G.; Burgos-Hernandez, A.; Calderon-Santoyo, M.; Lopez-Saiz, C.M.; Velazquez-Contreras, C.A.; Navarro-Ocana, A.; Ragazzo-Sanchez, J.A. Screening antimutagenic and antiproliferative properties of extracts isolated from Jackfruit pulp (Artocarpus heterophyllus Lam). Food Chem. 2015, 175, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Zhou, H.; George, S.; Hay, C.; Lee, J.; Qian, H.; Sun, X. Individual and combined effects of Aflatoxin B1, Deoxynivalenol and Zearalenone on HepG2 and RAW 264.7 cell lines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 103, 18–27. [Google Scholar] [CrossRef]
- Belguith-Hadriche, O.; Ammar, S.; Contreras, M.D.M.; Fetoui, H.; Segura-Carretero, A.; El Feki, A.; Bouaziz, M. HPLC-DAD-QTOF-MS profiling of phenolics from leaf extracts of two Tunisian fig cultivars: Potential as a functional food. Biomed. Pharmacother. 2017, 89, 185–193. [Google Scholar] [CrossRef]
- Diaz-Garcia, M.C.; Obon, J.M.; Castellar, M.R.; Collado, J.; Alacid, M. Quantification by UHPLC of total individual polyphenols in fruit juices. Food Chem. 2013, 138, 938–949. [Google Scholar] [CrossRef]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MS(n) and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Seo, O.N.; Kim, G.-S.; Kim, Y.-H.; Park, S.; Jeong, S.W.; Lee, S.J.; Jin, J.S.; Shin, S.C. Determination of polyphenol components of Korean Scutellaria baicalensis Georgi using liquid chromatography–tandem mass spectrometry: Contribution to overall antioxidant activity. J. Funct. Foods 2013, 5, 1741–1750. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Zarena, A.S.; Udaya Sankar, K. Supercritical carbon dioxide extraction of xanthones with antioxidant activity from Garcinia mangostana: Characterization by HPLC/LC–ESI-MS. J. Supercrit. Fluid. 2009, 49, 330–337. [Google Scholar] [CrossRef]
- Mekky, R.H.; del Mar Contreras, M.; El-Gindi, M.R.; Abdel-Monem, A.R.; Abdel-Sattar, E.; Segura-Carretero, A. Profiling of phenolic and other compounds from Egyptian cultivars of chickpea (Cicer arietinum L.) and antioxidant activity: A comparative study. RSC Adv. 2015, 5, 17751–17767. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macia, A.; Romero, M.P.; Motilva, M.J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.; Ranaweera, K.; Rupasinghe, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Zhang, L.; Su, H.; Zheng, X. Blackberry subjected to in vitro gastrointestinal digestion affords protection against Ethyl Carbamate-induced cytotoxicity. Food Chem. 2016, 212, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Spinola, V.; Llorent-Martinez, E.J.; Castilho, P.C. Antioxidant polyphenols of Madeira sorrel (Rumex maderensis): How do they survive to in vitro simulated gastrointestinal digestion? Food Chem. 2018, 259, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, Y.; Lou, S.; Li, H.; Ye, X. In vitro digestion combined with cellular assay to determine the antioxidant activity in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits: A comparison with traditional methods. Food Chem. 2014, 146, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bermudezsoto, M.; Tomasbarberan, F.; Garciaconesa, M. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, S.; Baum, M.; Eisenbrand, G.; Janzowski, C. Modulation of oxidative cell damage by reconstituted mixtures of phenolic apple juice extracts in human colon cell lines. Mol. Nutr. Food Res. 2006, 50, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Unit | JFE | JFG0 | JFG | |
|---|---|---|---|---|
| Total phenolic content (TPC) | mg GAE/mL | 2.089 ± 0.13 a | 1.665 ± 0.06 b | 0.6660 ± 0.04 c |
| Antioxidant capacity (AC) | μmol Fe2+/mL | 0.622 ± 0.02 a | 0.5644 ± 0.02 b | 0.3050 ± 0.02 c |
| AC/TPC | % | 29.70% | 33.90% | 45.80% |
| Peak | RT(min) | Molecular Weight | Possible Compounds |
|---|---|---|---|
| Increase | |||
| 1 | 1.648 | 131.1125 | Unknown |
| 2 | 2.063 | 165.1037 | Unknown |
| 3 | 2.43 | 230.17 | Prenyl-7-hydroxy |
| 4 | 2.669 | 246.1297 | Marmesin isomer |
| 5 | 6.964 | 211.1187 | Unknown |
| 6 | 7.66 | 554.3432 | Caffeic acid derivative |
| 7 | 11.902 | 564.3926 | Apigenin-6-C-glucosyl-8 carabinoside |
| 8 | 14.373 | 406.2784 | Citric acid derivative |
| 9 | 15.761 | 816.7375 | Unknown |
| 10 | 18.965 | 356.2823 | Ferulic acid-O-hexoside |
| 11 | 19.428 | 356.2794 | Feruloylglucoside |
| Decrease | |||
| 12 | 4.401 | 354.0991 | Caffeoylquinic acid |
| 13 | 5.089 | 376.1074 | Skullcapflavon |
| 14 | 8.397 | 353.1739 | Neochlorogenic |
| 15 | 9.437 | 451.1758 | Unknown |
| 16 | 10.54 | 396.1404 | Gartanin |
| 17 | 23.763 | 278.1612 | Unknown |
| No significant change | |||
| 18 | 25.853 | 403.2346 | Dihydroxybenzoic acid malonyl hexoside |
| 19 | 27.667 | 256.2659 | Unknown |
| 20 | 21.41 | 326.0776 | p-Coumaric acid-O-hexoside |
| 21 | 28.334 | 282.2782 | Unknown |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, D.; Liu, C.; Jiang, M.; Feng, L.; Chen, Y.; Han, J. After In Vitro Digestion, Jackfruit Flake Affords Protection against Acrylamide-Induced Oxidative Damage. Molecules 2019, 24, 3322. https://doi.org/10.3390/molecules24183322
Qu D, Liu C, Jiang M, Feng L, Chen Y, Han J. After In Vitro Digestion, Jackfruit Flake Affords Protection against Acrylamide-Induced Oxidative Damage. Molecules. 2019; 24(18):3322. https://doi.org/10.3390/molecules24183322
Chicago/Turabian StyleQu, Daofeng, Chu Liu, Mengxue Jiang, Lifang Feng, Yuewen Chen, and Jianzhong Han. 2019. "After In Vitro Digestion, Jackfruit Flake Affords Protection against Acrylamide-Induced Oxidative Damage" Molecules 24, no. 18: 3322. https://doi.org/10.3390/molecules24183322
APA StyleQu, D., Liu, C., Jiang, M., Feng, L., Chen, Y., & Han, J. (2019). After In Vitro Digestion, Jackfruit Flake Affords Protection against Acrylamide-Induced Oxidative Damage. Molecules, 24(18), 3322. https://doi.org/10.3390/molecules24183322