Phytochemical Investigation of Tradescantia Albiflora and Anti-Inflammatory Butenolide Derivatives
Abstract
:1. Introduction
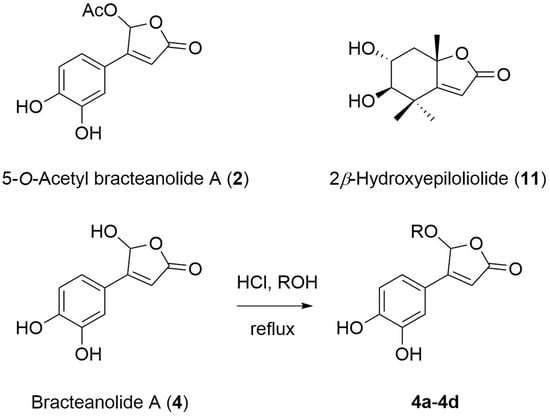
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Preparation of Butenolide Derivatives 4a–4d
3.5. Cell Culture
3.6. Cell Viability
3.7. Measurement of Nitric Oxide/Nitrite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.L.; Sheu, S.Y.; Huang, W.D.; Chuang, Y.L.; Tseng, H.C.; Hwang, T.S.; Fu, Y.T.; Kuo, Y.H.; Yao, C.H.; Kuo, T.F. Phytochemicals from Tradescantia albiflora Kunth extracts reduce serum uric acid levels in oxonate-induced rats. Pharm. Mag. 2016, 12, S223–S227. [Google Scholar]
- Wang, G.J.; Chen, S.M.; Chen, W.C.; Chang, Y.M.; Lee, T.H. Selective inducible nitric oxide synthase suppression by new bracteanolides from Murdannia bracteata. J. Ethnopharmacol. 2007, 112, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.H.; Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Nguyen, T.H.; Woo, M.H. Protein tyrosine phosphatase 1B (PTP1B) inhibitory constituents from the aerial parts of Tradescantia spathacea Sw. Fitoterapia 2015, 103, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wen, H.; Cui, Y.; Fan, M.; Liu, Z.; Mei, L.; Shao, Y.; Wang, Y.; Tao, Y. Phenolics from Lagotis brevituba Maxim. Nat. Prod. Res. 2017, 31, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ziosi, P.; Paolucci, C.; Santarelli, F.; Tabanelli, T.; Passeri, S.; Cavani, F.; Righi, P. A two-step process for the synthesis of hydroxytyrosol. ChemSusChem. 2018, 11, 2202–2210. [Google Scholar] [CrossRef]
- Govindan, B.; Johnson, A.J.; Viswanathan, G.; Ramaswamy, V.; Koshy, K.C.; Baby, S. Secondary metabolites from the unique bamboo, Melocanna baccifera. Nat. Prod. Res. 2019, 33, 122–125. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- De Marino, S.; Borbone, N.; Gala, F.; Zollo, F.; Fico, G.; Pagiotti, R.; Iorizzi, M. New constituents of sweet Capsicum annuum L. fruits and evaluation of their biological activity. J. Agric. Food Chem. 2006, 54, 7508–7516. [Google Scholar] [CrossRef]
- Yamano, Y.; Sasaki, H.; Wada, A. Versatile amine-promoted mild methanolysis of 3,5-dinitrobenzoates and its application to the synthesis of colorado potato beetle pheromone. Chem. Pharm. Bull. 2017, 65, 940–944. [Google Scholar] [CrossRef]
- Xiong, H.P.; Mi, J.L.; Le, J.M.; Wu, Z.J.; Chen, W.S. Chemical constituents of Ampelopsis japonica. Chem. Nat. Compd. 2017, 53, 791–793. [Google Scholar] [CrossRef]
- Clemente-Tejeda, D.; Bermejo, F.A. Oxidation of alkenes with non-heme iron complexes: Suitability as an organic synthetic method. Tetrahedron 2014, 70, 9381–9386. [Google Scholar] [CrossRef]
- Jia, X.; Yang, D.; Yang, Y.; Xie, H. Carotenoid-derived flavor precursors from Averrhoa carambola fresh fruit. Molecules 2019, 24, 256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, W.; Yang, X.; Xiu, F.; Xu, H.; Ying, X.; Stien, D. An isoindole alkaloid from Portulaca oleracea L. Nat. Prod. Res. 2018, 32, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.; Karym el, M.; Zarrouk, A.; Nury, T.; El Kharrassi, Y.; Nasser, B.; Cherkaoui Malki, M.; Lizard, G.; Samadi, M. An expeditious synthesis of spinasterol and schottenol, two phytosterols present in argan oil and in cactus pear seed oil, and evaluation of their biological activities on cells of the central nervous system. Steroids 2015, 99, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Soroka, D.; Sang, S. Oxyphytosterols as active ingredients in wheat bran suppress human colon cancer cell growth: Identification, chemical synthesis, and biological evaluation. J. Agric. Food Chem. 2015, 63, 2264–2276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Ren, W.; Zhao, D.; Zhu, Y.; Wu, X. Bioactive metabolites from Chaetomium globosum L18, an endophytic fungus in the medicinal plant Curcuma wenyujin. Phytomedicine 2012, 19, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Liu, T.; Gu, C.X.; Shao, C.L.; Zhou, J.; Wang, C.Y. Steroids and triterpenoids from the brown alga Kjellmaniella crassifolia. Chem. Nat. Compd. 2012, 48, 158–160. [Google Scholar] [CrossRef]
- Jeong, G.H.; Cho, J.H.; Jo, C.; Lee, S.; Lee, S.S.; Bai, H.W.; Chung, B.Y.; Kim, T.H. Gamma irradiation-assisted degradation of rosmarinic acid and evaluation of structures and anti-adipogenic properties. Food Chem. 2018, 258, 181–188. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, J.J.; Huang, H.C.; Huang, G.J.; Wang, S.Y.; Sung, P.J.; Cheng, M.J.; Wu, M.D.; Kuo, Y.H. New Benzenoid Derivatives and Other Constituents from Lawsonia inermis with Inhibitory Activity against NO Production. Molecules 2017, 22, 936. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Hamalainen, M.; Kankaanranta, H.; Moilanen, E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharm. 2002, 62, 698–704. [Google Scholar] [CrossRef]
- Bordag, N.; Klie, S.; Jurchott, K.; Vierheller, J.; Schiewe, H.; Albrecht, V.; Tonn, J.C.; Schwartz, C.; Schichor, C.; Selbig, J. Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Sci. Rep. 2015, 5, 15954. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–10 and 18–28 are available from the authors. |



| 1 a | 2 b | |||
|---|---|---|---|---|
| No. | δH | δC | δH | δC |
| 2 | 179.8 | 171.0 | ||
| 3 | 2.86 dd (17.4, 8.6) 2.62 dd (17.4, 8.9) | 36.9 | 6.49 s | 112.8 |
| 4 | 3.68 m | 42.0 | 162.8 | |
| 5 | 4.62 t (8.0) 4.20 t (8.0) | 76.2 | 7.40 s | 93.5 |
| 1’ | 133.0 | 121.6 | ||
| 2’ | 6.71 d (2.0) | 115.0 | 7.15 d (2.1) | 115.3 |
| 3’ | 146.9 | 146.8 | ||
| 4’ | 145.9 | 150.7 | ||
| 5’ | 6.73 d (8.4) | 116.8 | 6.94 d (8.3) | 116.8 |
| 6’ | 6.61 dd (8.4, 2.0) | 119.3 | 7.11 dd (8.3, 2.1) | 121.7 |
| 1’’ | 170.0 | |||
| 2’’ | 2.15 s | 20.8 | ||
| OH | 8.56 s | |||
| 11 | ||
|---|---|---|
| No. | δH | δC |
| 1 | 39.6 | |
| 2 | 3.04 d (9.3) | 81.7 |
| 3 | 3.80 ddd (12.1, 9.3, 4.4) | 67.9 |
| 4 | 1.43 t (12.1) 2.38 dd (12.1, 4.4) | 43.6 |
| 5 | 85.3 | |
| 6 | 180.3 | |
| 7 | 5.78 s | 114.0 |
| 8 | 170.7 | |
| 9 | 1.33 s | 25.7 |
| 10 | 1.18 s | 18.7 |
| 11 | 1.58 s | 25.5 |
| Compounds | IC50 (μg/mL) |
|---|---|
| 2 | 37.48 ± 1.38 |
| 3 | 18.75 ± 3.37 |
| 4 | 10.11 ± 0.35 |
| 4a | 21.77 ± 1.67 |
| 4b | 9.97 ± 0.32 |
| 4c | 22.74 ± 3.56 |
| 4d | 4.32 ± 0.09 |
| 5 | 16.51 ± 0.81 |
| 6 | 8.93 ± 1.06 |
| 7 | 5.76 ± 0.11 |
| 8 | >50 |
| Dexamethasone | 0.97 ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, P.-C.; Tseng, H.-C.; Liang, Y.-C.; Huang, G.-J.; Lu, T.-L.; Kuo, T.-F.; Kuo, Y.-H. Phytochemical Investigation of Tradescantia Albiflora and Anti-Inflammatory Butenolide Derivatives. Molecules 2019, 24, 3336. https://doi.org/10.3390/molecules24183336
Tu P-C, Tseng H-C, Liang Y-C, Huang G-J, Lu T-L, Kuo T-F, Kuo Y-H. Phytochemical Investigation of Tradescantia Albiflora and Anti-Inflammatory Butenolide Derivatives. Molecules. 2019; 24(18):3336. https://doi.org/10.3390/molecules24183336
Chicago/Turabian StyleTu, Ping-Chen, Han-Chun Tseng, Yu-Chia Liang, Guan-Jhong Huang, Te-Ling Lu, Tzong-Fu Kuo, and Yueh-Hsiung Kuo. 2019. "Phytochemical Investigation of Tradescantia Albiflora and Anti-Inflammatory Butenolide Derivatives" Molecules 24, no. 18: 3336. https://doi.org/10.3390/molecules24183336
APA StyleTu, P. -C., Tseng, H. -C., Liang, Y. -C., Huang, G. -J., Lu, T. -L., Kuo, T. -F., & Kuo, Y. -H. (2019). Phytochemical Investigation of Tradescantia Albiflora and Anti-Inflammatory Butenolide Derivatives. Molecules, 24(18), 3336. https://doi.org/10.3390/molecules24183336