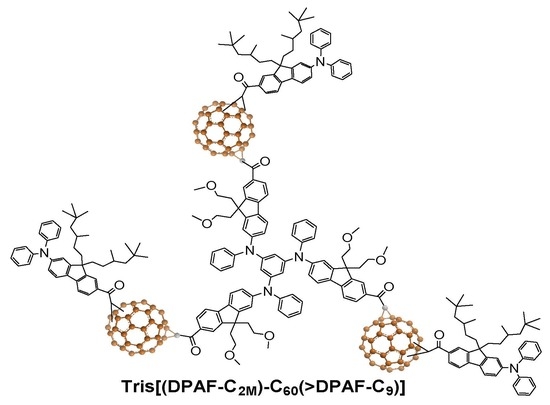
Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Characterization of Synthetic 3D Configurated Fullerenyl Nanomaterials
2.2. Photophysical and Physical Properties of 3D Conformeric Fullerenyl Nanomaterials
2.3. Evidence of Intramolecular Energy- and Electron-Transfer Events within cis-cup-4-C2M–9 by Detection of Corresponding Reactive Oxygen Species (ROS)
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Instruments for Spectroscopic Measurements
3.3. Synthesis of N1,N3,N5-Tris(9,9-di(methoxyethyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)-benzene as Tris(DPAF-C2M) (2-C2M)
3.4. Synthesis of N1,N3,N5-Tris(7-α-bromoacetyl-9,9-di(methoxyethyl)fluoren-2-yl)-1″,3″,5″-tris-(phenylamino)benzene as Tris(BrDPAF-C2M) (3-C2M)
3.5. Synthesis of N1,N3,N5-Tris(7-(1,2-dihydro-1,2-methanofullerene[60]-61-carbonyl)-9,9-di(methoxyethyl)fluoren-2-yl)-1″,3″,5″-tris(phenylamino)benzene) as Tris[(DPAF-C2M)-C60(>DPAF-C9)] (4-C2M–9)
3.6. ROS Measurements Using singlet oxygen (1O2)-Sensitive Fluorescent Probe
3.7. ROS Measurements Using Superoxide Radical (O2−·)-Sensitive Fluorescent Probe
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Khouly, M.E.; Ito, O. Intermolecular and supramolecular photoinduced electron transfer processes of fullerene–porphyrin/phthalocyanine systems. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 79–104. [Google Scholar]
- Escudero, D. Revising intramolecular photoinduced electron transfer (PET) from first-principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Ito, O.; D’Souza, F. Recent advances in photoinduced electron transfer processes of fullerene-based molecular assemblies and nanocomposites. Molecules 2012, 17, 5816–5835. [Google Scholar] [PubMed]
- Wu, W.; Zhao, J.; Sun, J.; Guo, S. Light-harvesting fullerene dyads as organic triplet photosensitizers for triplet–triplet annihilation upconversions. J. Org. Chem. 2012, 77, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Allen, B.D.; Rewinska, D.B.; Harriman, A. Selective triplet-state formation during charge recombination in a fullerene/bodipy molecular dyad (bodipy=borondipyrromethene). Chem. Eur. J. 2009, 15, 7382–7393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Chea, Y.; Yuan, X.; Cai, F.; Zhaoa, J.; Zhaoa, X.; Xub, H.; Liu, L. Bodipy−corrole dyad with truxene bridge: Photophysical properties and application in triplet−triplet annihilation upconversion. Dyes Pigments 2019, 171, 107756. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Natali, M.; Campagna, S.; Scandola, F. Photoinduced electron transfer across molecular bridges: Electron- and hole-transfer superexchange pathways. Chem. Soc. Rev. 2014, 43, 4005–4018. [Google Scholar] [CrossRef]
- Imahori, H.; Sakata, Y. Donor-linked fullerenes: Photoinduced electron transfer and its potential application. Adv. Mater. 1997, 9, 537–546. [Google Scholar]
- D’Souza, F.; Ito, O. Photosensitized electron transfer processes of nanocarbons applicable to solar cells. Chem. Soc. Rev. 2012, 41, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Torre, G.; Guldi, D.M.; Torres, T. Covalent and noncovalent phthalocyanine-carbon nanostructure systems: Synthesis, photoinduced electron transfer, and application to molecular photovoltaics. Chem. Rev. 2010, 110, 6768–6816. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Ling, J.; Prasanna de Silva, A. Current developments in fluorescent PET (photoinduced electron transfer) sensors and switches. Chem. Soc. Rev. 2015, 44, 4203–4211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with decacationic [60]fullerene monoadducts: Effect of a light absorbing e−-donor antenna and micellar formulation. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, M.; Huang, Y.-Y.; Landi, G.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: Oxygen independent photokilling in presence of azide and new mechanistic insights. Free Radic. Biol. Med. 2014, 79, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Martin, N. [60]Fullerene dimer. Chem. Soc. Rev. 2000, 29, 13–25. [Google Scholar] [CrossRef]
- Shirai, Y.; Osgood, A.J.; Zhao, Y.; Kelly, K.F.; Tour, J.M. Directional control in thermally driven single-molecule nanocars. Nano Lett. 2005, 5, 2330–2334. [Google Scholar] [CrossRef]
- Akimov, A.V.; Nemukhin, A.V.; Moskovsky, A.A.; Kolomeisky, A.B.; Tour, J.M. Molecular dynamics of surface-moving thermally driven nanocars. J. Chem. Theory Comput. 2008, 4, 652–656. [Google Scholar] [CrossRef]
- Sasaki, T.; Osgood, A.J.; Kiappes, J.L.; Kelly, K.F.; Tour, J.M. Synthesis of a porphyrin-fullerene pinwheel. Org. Lett. 2008, 10, 1377–1380. [Google Scholar] [CrossRef]
- Zhang, J.; Porfyrakis, K.; Morton, J.J.L.; Sambrook, M.R.; Harmer, J.; Xiao, L.; Ardavan, A.; Briggs, G.A.D.; Briggs, G. Photoisomerization of a fullerene dimer. J. Phys. Chem. 2008, C 112, 2802–2904. [Google Scholar] [CrossRef]
- Wang, J.L.; Duan, X.F.; Jiang, B.; Gan, L.B.; Pei, J.; He, C.; Li, Y.F. Nanosized rigid π-conjugated molecular heterojunctions with multi[6 0]fullerenes: Facile synthesis and photophysical properties. J. Org. Chem. 2006, 71, 4400–4410. [Google Scholar] [CrossRef] [PubMed]
- Lόpez-Andarias, J.; Bauza, A.; Sakai, N.; Frontera, A.; Matile, S. Remote control of anion-π catalysis on fullerene-centered catalytic triads. Angew. Chem. 2018, 130, 11049–11053. [Google Scholar] [CrossRef]
- Sabirov, D.S. Polarizability of C60 fullerene dimer and oligomers: The unexpected enhancement and its use for rational design of fullerene-based nanostructures with adjustable properties. RSC Adv. 2013, 3, 19430. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tukhbatullina, A.A.; Sabirov, D.S. Dipole polarizability, structure, and stability of [2+2]-linked fullerene nanostructures (C60)n (n ≤ 7). Phys. E Low-Dimens. Syst. Nanostruct. 2017, 86, 237–242. [Google Scholar] [CrossRef]
- Tukhbatullina, A.; Shepelevich, I.; Sabirov, D.S. Exaltation of polarizability as a common property of fullerene dimers with divers intercage bridges. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 661–666. [Google Scholar] [CrossRef]
- Swart, M.; van Duijnen, P.T. Rapid determination of polarizability exaltation in fullerene-based nanostructures. J. Mater. Chem. 2015, C3, 23–25. [Google Scholar] [CrossRef]
- Wang, M.; Su, C.; Yu, T.; Tan, L.-S.; Hu, B.; Urbas, A.; Chiang, L.Y. Novel photoswitchable dielectric properties on nanomaterials of electronic core-shell γ-FeOx@Au@fullerosomes for GHz frequency applications. Nanoscale 2016, 8, 6589–6599. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.; Chiang, L.Y. Tunability of rf-responses by plasmonic dielectric amplification using branched e−-polarizable C60-adducts on magnetic nanoparticles. J. Phys. Chem. C 2016, 120, 17711–17721. [Google Scholar] [CrossRef]
- Wang, M.; Yu, T.; Tan, L.-S.; Urbas, A.; Chiang, L.Y. Enhancement of photoswitchable dielectric property by conducting electron donors on plasmonic core-shell gold-fluorenyl C60 nanoparticles. J. Phys. Chem. C 2018, 122, 12512–12523. [Google Scholar] [CrossRef]
- Padmawar, P.A.; Rogers, J.O.; He, G.S.; Chiang, L.Y.; Canteenwala, T.; Tan, L.-S. Large cross-section enhancement and intramolecular energy transfer upon multiphoton absorption of hindered diphenylaminofluorene-C60 dyads and triads. Chem. Mater. 2006, 18, 4065–4074. [Google Scholar] [CrossRef]
- Padmawar, P.A.; Canteenwala, T.; Tan, L.-S.; Chiang, L.Y. Synthesis and characterization of photoresponsive diphenylaminofluorene chromophore adducts of [60]fullerene. J. Mater. Chem. 2006, 16, 1366–1378. [Google Scholar] [CrossRef]
- Luo, H.; Fujitsuka, M.; Araki, Y.; Ito, O.; Padmawar, P.; Chiang, L.Y. Inter- and intramolecular photoinduced electron-transfer processes between C60 and diphenylaminofluorene in solutions. J. Phys. Chem. B 2003, 107, 9312–9318. [Google Scholar] [CrossRef]
- Hu, R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.; Wong, K.S.; Pena-Cabrera, E.; et al. Twisted intramolecular charge transfer and aggregation-induced emission of BODIPY derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Kang, N.-G.; Kokubo, K.; Jeon, S.; Wang, M.; Lee, C.-L.; Canteenwala, T.; Tan, L.-S.; Chiang, L. Synthesis and photoluminescent properties of geometrically hindered cis-tris(diphenyl-aminofluorene) as precursors to light-emitting devices. Molecules 2015, 20, 4635–4654. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Wang, M.; Kokubo, K.; Kang, N.-G.; Wolf, L.; Tan, L.-S.; Chen, C.-T.; Chiang, L. New 3D-stereoconfigurated cis-tris(fluorenylphenylamino)-benzene with large steric hindrance to minimize π–π stacking in thin-film devices. Dyes Pigments 2018, 149, 377–386. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Padmawar, P.A.; Canteenwala, T.; Tan, L.-S.; He, G.S.; Kannan, R.; Vaia, R.; Lin, T.-C.; Zheng, Q.; Prasad, P.N. Synthesis of C60-diphenylaminofluorene dyad with large 2PA cross-sections and efficient intramolecular two-photon energy transfer. Chem. Commun. 2002, 1854–1855. [Google Scholar] [CrossRef]
- Jeon, S.; Wang, M.; Ji, W.; Tan, L.-S.; Cooper, T.; Chiang, L.Y. Broadband two-photon absorption characteristics of highly photostable fluorenyl-dicyanoethylenylated [60]fullerene dyads. Molecules 2016, 21, 647. [Google Scholar] [CrossRef]
- Maeda, H.; Yamamoto, K.; Nomura, Y.; Kohno, I.; Hafsi, L.; Ueda, N.; Yoshida, S.; Fukuda, M.; Fukuyasu, Y.; Yamauchi, Y.; et al. A design of fluorescent probes for superoxide based on a nonredox mechanism. J. Am. Chem. Soc. 2005, 127, 68–69. [Google Scholar] [CrossRef]
- Wang, M.; Huang, L.; Sharma, S.K.; Jeon, S.; Thota, S.; Sperandio, F.F.; Nayka, S.; Chang, J.; Hamblin, M.R.; Chiang, L.Y. Synthesis and photodynamic effect of new highly photostable decacationically armed [60]- and [70]fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J. Med. Chem. 2012, 55, 4274–4285. [Google Scholar] [CrossRef]
- Wang, M.; Maragani, S.; Huang, L.; Jeon, S.; Canteenwala, T.; Hamblin, M.R.; Chiang, L.Y. Synthesis of decacationic [60]fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactvation. Eur. J. Med. Chem. 2013, 63, 170–184. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Sharma, S.K.; Wang, M.; Jeon, S.; Huang, Y.-Y.; Dai, T.; Nayka, S.; de Sousa, S.C.O.M.; Chiang, L.Y.; Hamblin, M.R. Photoinduced electron-transfer mechanisms for radical-enhanced photodynamic therapy mediated by water-soluble decacationic C70 and C84O2 fullerene derivatives. Nanomed. Nanotech. Biol. Med. 2013, 9, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, M.; Huang, Y.-Y.; El-Hussein, A.; Chiang, L.Y.; Hamblin, M.R. Progressive cationic functionalization of chlorin derivatives for antimicrobial photodynamic inactivation and related vancomycin conjugate. Photochem. Photobiol. Sci. 2018, 17, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, M.; Yu, T.; Tan, L.-S.; Chiang, L.Y. 3D-conformer of tris[60]fullerenylated cis-tris(diphenylaminofluorene) as photoswitchable charge-polarizer on GHz-responsive trilayered core-shell dielectric nanoparticles. Molecules 2018, 23, 1873. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Haley, J.; Flikkema, J.; Nalla, V.; Wang, M.; Sfeir, M.; Tan, L.-S.; Cooper, T.; Ji, W.; Hamblin, M.R.; et al. Linear and nonlinear optical properties of light-harvesting hybrid [60]fullerene triads and tetraads with dual NIR two-photon absorption characteristics. J. Phys. Chem. C 2013, 117, 17186–17195. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of tris[(DPAF-C2M)-C60(>DPAF-C9)] is available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Wang, M.; Tan, L.-S.; Chiang, L.Y. Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives. Molecules 2019, 24, 3337. https://doi.org/10.3390/molecules24183337
Yin H, Wang M, Tan L-S, Chiang LY. Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives. Molecules. 2019; 24(18):3337. https://doi.org/10.3390/molecules24183337
Chicago/Turabian StyleYin, He, Min Wang, Loon-Seng Tan, and Long Y. Chiang. 2019. "Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives" Molecules 24, no. 18: 3337. https://doi.org/10.3390/molecules24183337
APA StyleYin, H., Wang, M., Tan, L. -S., & Chiang, L. Y. (2019). Synthesis and Intramolecular Energy- and Electron-Transfer of 3D-Conformeric Tris(fluorenyl-[60]fullerenylfluorene) Derivatives. Molecules, 24(18), 3337. https://doi.org/10.3390/molecules24183337