Understanding Urea Encapsulation in Different Clay Minerals as a Possible System for Ruminant Nutrition
Abstract
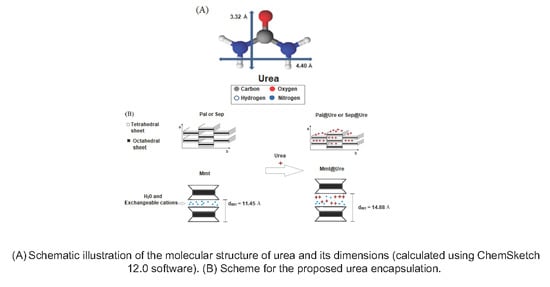
:1. Introduction
2. Results and Discussion
2.1. X-Ray Diffraction (XRD)
2.2. Thermogravimetric Analysis (TG-DTG)
2.3. Scanning Electronic Microscopy (SEM)
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Zeta Potential ζ
3. Materials and Methods
3.1. Materials
3.2. Urea Encapsulation into Clay Minerals
3.3. Characterizations
3.3.1. X-Ray Diffraction (XRD)
3.3.2. Thermogravimetric Analysis (TG-DTG)
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Infrared Spectroscopy (FTIR)
3.3.5. Zeta Potential
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Azevedo, E.B.; Patiño, H.O.; Da Silveira, A.L.F.; López, J.; Brüning, G.; Kozloski, G.V. Incorporação de uréia encapsulada em suplementos protéicos fornecidos para novilhos alimentados com feno de baixa qualidade. Cienc. Rural 2008, 38, 1381–1387. [Google Scholar] [CrossRef]
- Santos, F.A.P.; Pedroso, A.M. Metabolismo de proteínas. In Nutrição de Ruminantes, 2nd ed.; Berchielli, T.T., Pires, A.V., Oliveira, S.G., Eds.; FUNEP: Jaboticabal, Brazil, 2011; pp. 265–297. ISBN 85-87632-72-8. [Google Scholar]
- Bartley, E.E.; Deyoe, C.W. Starea as a protein replacer for ruminants. A review of 10 years of research. Feedstuffs 1975, 47, 42–44. [Google Scholar]
- Prokop, M.J.; Klopfenstein, T.J. Slow Ammonia Release Urea; Nebraska Beef Cattle Report No. EC 77-218; University of Nebraska–Lincoln: Lincoln, NE, USA, 1997. [Google Scholar]
- Forero, O.; Owens, F.N.; Lusby, K.S. Evaluation of slow-release urea for winter supplementation of lactating range cows. J. Anim. Sci. 1980, 50, 532–538. [Google Scholar] [CrossRef]
- Löest, C.A.; Lambert, B.D.; Trater, A.M.; Titgemeyer, E.C. Branched-chain amino acids for growing cattle limit-fed diets based on soybean hulls. Kansas Agric. Exp. Stn. Res. Rep. 2001, 79, 77–79. [Google Scholar]
- Cass, J.L.; Richardson, C.R. Vitro Ammonia Release from Urea/Calcium Compounds as Compared to Urea and Cottonseed Meal; Technical Report No. T-5-342; Texas Tech University: Lubbock, TX, USA, 1994. [Google Scholar]
- Galo, E.; Emanuele, S.M.; Sniffen, C.J.; White, J.H.; Knapp, J.R. Effects of a polymer-coated urea product on nitrogen metabolism in lactating holstein dairy cattle. J. Dairy Sci. 2003, 86, 2154–2162. [Google Scholar] [CrossRef]
- Todd, R.D. Microencapsulation and Flavour Industry; Flavour Industry: London, UK, 1970. [Google Scholar]
- Dubey, S.C.; Bhavani, R.; Singh, B. Development of Pusa 5SD for seed dressing and Pusa Biopellet 10G for soil application formulations of Trichoderma harzianum and their evaluation for integrated management of dry root rot of mungbean (Vigna radiata). Biol. Control 2009, 50, 231–242. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoparticle and Microparticle-Based Delivery Systems: Encapsulation, Protection and Release of Active Compounds, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781138034037. [Google Scholar]
- Ezhilarasi, P.N.; Indrani, D.; Jena, B.S.; Anandharamakrishnan, C. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. J. Food Eng. 2013, 117, 513–520. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar]
- Rosenberg, M.; Kopelman, I.J.; Talmon, Y. Factors affecting retention in spray-drying microencapsulation of volatile materials. J. Agric. Food Chem. 1990, 38, 1288–1294. [Google Scholar] [CrossRef]
- Jackson, L. Microencapsulation in the food industry. Lebensm. Wiss. Technol. 1991, 24, 289. [Google Scholar]
- Viseras, C.; Lopez-Galindo, A. Pharmaceutical applications of some Spanish clays (sepiolite, palygorskite, bentonite): Some preformulation studies. Appl. Clay Sci. 1999, 14, 69–82. [Google Scholar] [CrossRef]
- Hermosin, M.C.; Cornejo, J.; White, J.L.; Hem, S.L. Sepiolite, a potential excipient for drugs subject to oxidative degradation. J. Pharm. Sci. 1981, 70, 189–192. [Google Scholar] [CrossRef]
- Galán, E.; Forteza, M. Minerales utilizados en la industria. Boletín de la Sociedad Española de Mineralogía 1985, 8, 369–378. [Google Scholar]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl. Clay Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Santana, A.C.S.G.V.; Sobrinho, J.L.S.; da Silva Filho, E.C.; Nunes, L.C.C. Obtaining the palygorskite:chitosan composite for modified release of 5-aminosalicylic acid. Mater. Sci. Eng. C 2017, 73, 245–251. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.S.F.; Osajima, J.A.; Luz, A.B.; Fonseca, M.G.; Silva Filho, E.C. Thermally activated palygorskites as agents to clarify soybean oil. Appl. Clay Sci. 2016, 119, 338–347. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Li, S.X.; Hu, X.P.; Wang, P.J.; Zeng, J.B.; Wang, X.L.; Wang, Y.Z. Structure, morphology, and properties of LDPE/sepiolite nanofiber nanocomposite. Polym. Adv. Technol. 2017, 28, 958–964. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Wang, F.; Liang, J.; Ran, S.; Sun, J. Phase transformation and morphology evolution of sepiolite fibers during thermal treatment. Appl. Clay Sci. 2017, 143, 205–211. [Google Scholar] [CrossRef]
- dos Santos, A.; Viante, M.F.; Pochapski, D.J.; Downs, A.J.; Almeida, C.A.P. Enhanced removal of p-nitrophenol from aqueous media by montmorillonite clay modified with a cationic surfactant. J. Hazard. Mater. 2018, 355, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.F.; Oliveira, M.M.; Avelino, M.C.; Fonseca, M.G.; Almeida, R.K.S.; Silva Filho, E.C. Adsorption of an industrial anionic dye by modified-KSF-montmorillonite: Evaluation of the kinetic, thermodynamic and equilibrium data. Chem. Eng. J. 2012, 203, 259–268. [Google Scholar] [CrossRef]
- Gamba, M.; Kovář, P.; Pospíšil, M.; Torres Sánchez, R.M. Insight into thiabendazole interaction with montmorillonite and organically modified montmorillonites. Appl. Clay Sci. 2017, 137, 59–68. [Google Scholar] [CrossRef]
- Zhong, L.; Tang, A.; Wen, X.; Yan, P.; Wang, J.; Tan, L.; Chen, J. New finding on Sb (2–3 nm) nanoparticles and carbon simultaneous anchored on the porous palygorskite with enhanced catalytic activity. J. Alloys Compd. 2018, 743, 394–402. [Google Scholar] [CrossRef]
- Erdoğan Alver, B. Hydrogen adsorption on natural and sulphuric acid treated sepiolite and bentonite. Int. J. Hydrogen Energy 2018, 43, 831–838. [Google Scholar] [CrossRef]
- Alves, J.L.; de Rosa, P.T.V.; Morales, A.R. Evaluation of organic modification of montmorillonite with ionic and nonionic surfactants. Appl. Clay Sci. 2017, 150, 23–33. [Google Scholar] [CrossRef]
- Çağatay, M.N. Palygorskite in the Eocene rocks of the Dammam dome. Clays Clay Miner. 1990, 38, 299–307. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; He, H. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 153–161. [Google Scholar] [CrossRef]
- Luo, W.; Sasaki, K.; Hirajima, T. Influence of the pre-dispersion of montmorillonite on organic modification and the adsorption of perchlorate and methyl red anions. Appl. Clay Sci. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Viseras, C.; Cerezo, P.; Aguzzi, C. Adsorption and characterization of palygorskite-isoniazid nanohybrids. Appl. Clay Sci. 2018, 160, 180–185. [Google Scholar] [CrossRef]
- Madejová, J.; Sekeráková, U.; Bizovská, V.; Slaný, M.; Jankovič, L. Near-infrared spectroscopy as an effective tool for monitoring the conformation of alkylammonium surfactants in montmorillonite interlayers. Vib. Spectrosc. 2016, 84, 44–52. [Google Scholar] [CrossRef]
- Huo, C.; Yang, H. Synthesis and characterization of ZnO/palygorskite. Appl. Clay Sci. 2010, 50, 362–366. [Google Scholar] [CrossRef]
- Katti, K.S.; Katti, D.R.; Dash, R. Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed. Mater. 2008, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.A.; Nascimentos, S.Q.; Sousa, A.B.; Júnior, E.F.R.; Pereira, P.I.A.; Fontenele, V.M.; Silva Filho, E.C.; Magalhães, J.L.; Luz, R.A.S.; Catanhêde, W.; et al. Synthesis, characterization and electrochemical properties of composites synthesized from silver-tannic acid hybrid nanoparticles and different clays. Appl. Clay Sci. 2019, 181, 105219. [Google Scholar] [CrossRef]
- Silva, D.T.C.; Arruda, I.E.S.; França, L.M.; França, D.B.; Fonseca, M.G.; Soares, M.F.L.R.; Soares-Sobrinho, J.L. Tamoxifen/montmorillonite system—Effect of the experimental conditions. Appl. Clay Sci. 2019, 180, 105142. [Google Scholar] [CrossRef]
- Manivannan, M.; Rajendran, S. Investigation of Inhibitive Action of Urea- Zn 2 + System in the Corrosion Control of. Int. J. Eng. Sci. Technol. 2015, 3, 8048–8060. [Google Scholar]
- Machado, G.S.; Ucoski, G.M.; De Lima, O.J.; Ciuffi, K.J.; Wypych, F.; Nakagaki, S. Cationic and anionic metalloporphyrins simultaneously immobilized onto raw halloysite nanoscrolls catalyze oxidation reactions. Appl. Catal. A Gen. 2013, 460–461, 124–131. [Google Scholar] [CrossRef]
- De Faria, E.H.; Lima, O.J.; Ciuffi, K.J.; Nassar, E.J.; Vicente, M.A.; Trujillano, R.; Calefi, P.S. Hybrid materials prepared by interlayer functionalization of kaolinite with pyridine-carboxylic acids. J. Colloid Interface Sci. 2009, 335, 210–215. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







| Clay Minerals | Event 1 | Event 2 | Event 3 | Event 4 | |
|---|---|---|---|---|---|
| Palygorskite | Without Urea | 70 °C | 184 °C | 419 °C | 623 °C |
| 4.18% | 2.67% | 4.86% | 2.56% | ||
| With urea | 75 °C | 190 °C | 420 °C | 630 °C | |
| 4.71% | 2.72% | 4.74% | 2.61% | ||
| Sepiolite | Without Urea | 69 °C | 261 °C | 495 °C | 802 °C |
| 0.96% | 3.32% | 2.98% | 2.60% | ||
| With Urea | 71 °C | 268 °C | 496 °C | 812 °C | |
| 4.03% | 3.57% | 3.18% | 2.82% | ||
| Veegum® | Without Urea | 58 °C | 657 °C | 850 °C | - |
| 3.28% | 4.81% | 0.57% | - | ||
| With Urea | 59 °C | 667 °C | - | - | |
| 9.37% | 4.75% | - | - | ||
| Material | Zeta Potential of Clays (mV) | Zeta Potential of Clays with Urea (mV) |
|---|---|---|
| Palygorskite | −14.1 | −13.6 |
| Sepiolite | −22.0 | −18.7 |
| Veegum® | −34.4 | −32.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.C.; Lima, L.C.B.; Viseras, C.; Osajima, J.A.; da Silva Júnior, J.M.; Oliveira, R.L.; Bezerra, L.R.; Silva-Filho, E.C. Understanding Urea Encapsulation in Different Clay Minerals as a Possible System for Ruminant Nutrition. Molecules 2019, 24, 3525. https://doi.org/10.3390/molecules24193525
Silva FC, Lima LCB, Viseras C, Osajima JA, da Silva Júnior JM, Oliveira RL, Bezerra LR, Silva-Filho EC. Understanding Urea Encapsulation in Different Clay Minerals as a Possible System for Ruminant Nutrition. Molecules. 2019; 24(19):3525. https://doi.org/10.3390/molecules24193525
Chicago/Turabian StyleSilva, Fabrícia C., Luciano C. B. Lima, Cesar Viseras, Josy A. Osajima, Jarbas M. da Silva Júnior, Ronaldo L. Oliveira, Leilson R. Bezerra, and Edson C. Silva-Filho. 2019. "Understanding Urea Encapsulation in Different Clay Minerals as a Possible System for Ruminant Nutrition" Molecules 24, no. 19: 3525. https://doi.org/10.3390/molecules24193525
APA StyleSilva, F. C., Lima, L. C. B., Viseras, C., Osajima, J. A., da Silva Júnior, J. M., Oliveira, R. L., Bezerra, L. R., & Silva-Filho, E. C. (2019). Understanding Urea Encapsulation in Different Clay Minerals as a Possible System for Ruminant Nutrition. Molecules, 24(19), 3525. https://doi.org/10.3390/molecules24193525