An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides
Abstract
:1. Introduction
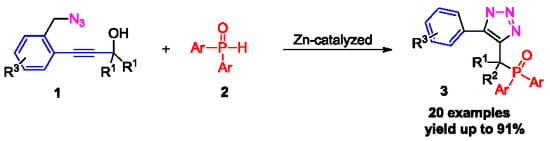
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berger, D.; Citarella, R.; Dutia, M.; Greenberger, L.; Hallett, W.; Paul, R.; Powell, D. Novel Multidrug Resistance Reversal Agents. J. Med. Chem. 1999, 42, 2145–2161. [Google Scholar] [CrossRef]
- Goethem, S.V.; Matheeussen, V.; Joossens, J.; Lambeir, A.-M.; Chen, X.; Meester, I.D.; Haemers, A.; Augustyns, K.; der Veken, P.V. Structure-Activity Relationship Studies on Isoindoline Inhibitors of Dipeptidyl Peptidases 8 and 9 (DPP8, DPP9): Is DPP8-Selectivity an Attainable Goal? J. Med. Chem. 2011, 54, 5737–5746. [Google Scholar] [CrossRef] [PubMed]
- Das Adhikary, N.; Chattopadhyay, P. Design and Synthesis of 1,2,3-Triazole-Fused Chiral Medium-Ring Benzo-Heterocycles, Scaffolds Mimicking Benzolactams. J. Org. Chem. 2012, 77, 5399–5405. [Google Scholar] [CrossRef] [PubMed]
- Kallander, L.S.; Lu, Q.; Chen, W.; Tomaszek, T.; Yang, G.; Tew, D.; Meek, T.D.; Hofmann, G.A.; Schulz-Pritchard, C.K.; Smith, W.W.; et al. 4-Aryl-1,2,3-triazole: A Novel Template for a Reversible Methionine Aminopeptidase 2 Inhibitor, Optimized To Inhibit Angiogenesis in Vivo. J. Med. Chem. 2005, 48, 5644–5647. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Harmatz, J.S.; Shapiro, L.; Engelhardt, N.; Gouthro, T.A.; Shader, R.I. Sensitivity to Triazolam in the Elderly. N. Engl. J. Med. 1991, 324, 1691–1698. [Google Scholar] [CrossRef]
- Tatsuta, K.; Ikeda, Y.; Miura, S. Synthesis and Glycosidase Inhibitory Activities of Nagstatin Triazole Analogs. J. Antibiot. 1996, 49, 836. [Google Scholar] [CrossRef] [PubMed]
- Hseih, H.-Y.; Lee, W.-C.; Chandru, G.C.; Hu, W.-P.; Liang, J.-J.; Tsai, T.-R.; Chou, Y.-W.; Kuo, K.-K.; Chen, C.-Y.; Wang, J.J.; et al. Discovery, Synthetic Methodology, and Biological Evaluation for Antiphotoaging Activity of Bicyclic[1,2,3]triazoles: In Vitro and in Vivo Studies. J. Med. Chem. 2013, 56, 5422–5435. [Google Scholar] [CrossRef]
- Shafran, E.A.; Bakulev, V.A.; Rozin, Y.A.; Shafran, Y.M. Condensed 1,2,3-triazoles. Chem. Heterocycl. Compd. 2008, 44, 1040–1069. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Ray, K. Synthesis of 1,2,3-Triazole-Fused Heterocycles via Intramolecular Azide-Alkyne Cycloaddition Reactions. Synthesis 2011, 3767–3783. [Google Scholar] [CrossRef]
- Choudhury, C.; Mandal, S.B.; Achari, B. Palladium-copper catalysed heteroannulation of acetylenic compounds: An expeditious synthesis of isoindoline fused with triazoles. Tetrahedron Lett. 2005, 46, 8531–8534. [Google Scholar] [CrossRef]
- Fiandanese, V.; Marchese, G.; Punzi, A.; Iannone, F.; Rafaschieri, G.C. An easy synthetic approach to 1,2,3-triazole-fused heterocycles. Tetrahedron 2010, 66, 8846–8853. [Google Scholar] [CrossRef]
- Fiandanese, V.; Marino, I.; Punzi, A. An easy access to 4-(1,2,3-triazolylalkyl)-1,2,3-triazole-fused dihydroisoquinolines and dihydroisoindoles. Tetrahedron 2012, 68, 10310–10317. [Google Scholar] [CrossRef]
- Brahma, K.; Achari, B.; Choudhury, C. Facile Synthesis of [1,2,3]-Triazole-Fused Isoindolines, Tetrahydroisoquinolines, Benzoazepines and Benzoazocines by Palladium-Copper Catalysed Heterocyclisation. Synthesis 2013, 45, 545–555. [Google Scholar]
- Ramachary, D.B.; Ramakumar, K.; Narayana, V.V. Amino Acid-Catalyzed Cascade [3+2]-Cycloaddition/Hydrolysis Reactions Based on the Push–Pull Dienamine Platform: Synthesis of Highly Functionalized NH-1,2,3-Triazoles. Chem. Eur. J. 2008, 14, 9143–9147. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Shashank, A.B. Organocatalytic Triazole Formation, Followed by Oxidative Aromatization: Regioselective Metal-Free Synthesis of Benzotriazoles. Chem. Eur. J. 2013, 19, 13175–13181. [Google Scholar] [CrossRef] [PubMed]
- Belkheira, M.; Abed, D.E.; Pons, J.-M.; Bressy, C. Organocatalytic Synthesis of 1,2,3-Triazoles from Unactivated Ketones and Arylazides. Chem. Eur. J. 2011, 17, 12917–12921. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, S.Y.; Danence, L.J.T.; Gao, Y.; Wang, J. Amine-Catalyzed [3+2] Huisgen Cycloaddition Strategy for the Efficient Assembly of Highly Substituted 1,2,3-Triazoles. Chem. Eur. J. 2012, 18, 6088–6093. [Google Scholar] [CrossRef]
- Seus, N.; Goncalves, L.C.; Deobald, A.M.; Savegnago, L.; Alves, D.; Paixao, M.W. Synthesis of arylselanyl-1H-1,2,3-triazole-4-carboxylates by organocatalytic cycloaddition of azidophenyl arylselenides with β-keto-esters. Tetrahedron 2012, 68, 10456–10463. [Google Scholar] [CrossRef]
- Li, W.; Jia, Q.; Du, Z.; Wang, J. Direct access to triazole-olefins through catalytic cycloaddition of azides to unsaturated aldehydes. Chem. Commun. 2013, 49, 10187–10189. [Google Scholar]
- Yeung, D.K.J.; Gao, T.; Huang, J.; Sun, S.; Guo, H.; Wang, J. Organocatalytic 1,3-dipolar cycloaddition reactions of ketones andazides with water as a solvent. Green Chem. 2013, 15, 2384–2388. [Google Scholar] [CrossRef]
- Seus, N.; Goldani, B.; Lenardão, E.J.; Savegnago, L.; Paixão, M.W.; Alves, D. Organocatalytic Synthesis of (Arylselanyl)phenyl-1H-1,2,3-triazole-4-carboxamides by Cycloaddition between Azidophenyl Arylselenides and β-Oxo-amides. Eur. J. Org. Chem. 2013, 2014, 1059–1065. [Google Scholar] [CrossRef]
- Li, W.; Du, Z.; Huang, J.; Jia, Q.; Zhang, K.; Wang, J. Direct access to 1,2,3-triazoles through organocatalytic 1,3-dipolar cycloaddition reaction of allyl ketones with azides. Green Chem. 2014, 16, 3003–3006. [Google Scholar] [CrossRef]
- Li, W.; Du, Z.; Zhang, K.; Wang, J. Organocatalytic 1,3-dipolar cycloaddition reaction of α,β-unsaturated ketones with azides through iminium catalysis. Green Chem. 2015, 17, 781–784. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Shashank, A.B.; Karthik, S. An Organocatalytic Azide–Aldehyde [3+2] Cycloaddition: High-Yielding Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2014, 53, 10420–10424. [Google Scholar] [CrossRef] [PubMed]
- Shashank, A.B.; Karthik, S.; Madhavachary, R.; Ramachary, D.B. An Enolate-Mediated Organocatalytic Azide–Ketone [3+2]-Cycloaddition Reaction: Regioselective High-Yielding Synthesis of Fully Decorated 1,2,3-Triazoles. Chem. Eur. J. 2014, 20, 16877–16881. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J. Lewis Base Catalyzed Aerobic Oxidative Intermolecular Azide–Zwitterion Cycloaddition. Angew. Chem. Int. Ed. 2014, 53, 14186–14190. [Google Scholar] [CrossRef] [PubMed]
- Ramasastry, S.S.V. Enamine/Enolate-Mediated Organocatalytic Azide–Carbonyl [3+2] Cycloaddition Reactions for the Synthesis of Densely Functionalized 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2014, 53, 14310–14312. [Google Scholar] [CrossRef]
- Ali, A.; Corrêa, A.G.; Alves, D.; Zukerman-Schpector, J.; Westermann, B.; Ferreira, M.A.B.; Paixao, M.W. An efficient one-pot strategy for the highly regioselective metal-free synthesis of 1,4-disubstituted-1,2,3-triazoles. Chem. Commun. 2014, 50, 11926–11929. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3070. [Google Scholar] [CrossRef]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [Green Version]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Garner, R.C.; Nicholson, S.; Kissling, C.J.; Mayers, D. Microdose Pharmacokinetics of IDX899 and IDX989, Candidate HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors, Following Oral and Intravenous Administration in Healthy Male Subjects. J. Clin. Pharmacol. 2009, 49, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Coluccia, A.; Silvestri, R. Antiviral. Chem. Chemother. 2010, 20, 213–237. [Google Scholar]
- Alexandre, F.R.; Amador, A.; Bot, S.; Caillet, C.; Convard, T.; Jakubik, J.; Musiu, C.; Poddesu, B.; Vargiu, L.; Liuzzi, M.; et al. Synthesis and Biological Evaluation of Aryl-phospho-indole as Novel HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. J. Med. Chem. 2011, 54, 392–395. [Google Scholar] [CrossRef]
- Muzart, J. Gold-catalysed reactions of alcohols: Isomerisation, inter- and intramolecular reactions leading to C–C and C–heteroatom bonds. Tetrahedron 2008, 64, 5815–5849. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Yao, M.-L. Direct Propargylic Substitution of Hydroxyl Group in Propargylic Alcohols. Curr. Org. Synth. 2008, 5, 28–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Lu, P.; Wang, Y. Recent Advances on the Lewis Acid-Catalyzed Cascade Rearrangements of Propargylic Alcohols and Their Derivatives. ACS Catal. 2014, 4, 1911–1925. [Google Scholar] [CrossRef]
- Song, X.-R.; Qiu, Y.-F.; Liu, X.-Y.; Liang, Y.-M. Recent advances in the tandem reaction of azides with alkynes or alkynols. Org. Biomol. Chem. 2016, 14, 11317–11331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Regioselective Rapid Synthesis of Fully Substituted 1,2,3-Triazoles Mediated by Propargyl Cations. Org. Lett. 2013, 15, 5222–5225. [Google Scholar] [CrossRef]
- Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Acid-mediated synthesis of fully substituted 1,2,3-triazoles: Multicomponent coupling reactions, mechanistic study, synthesis of serine hydrolase inhibitor and its derivatives. Tetrahedron 2014, 70, 9828–9835. [Google Scholar] [CrossRef]
- Yang, T.; Ding, H.; Li, R.; Jin, F.; Song, X.-R.; Chen, X.; Bai, J.; Xiao, Q.; Liang, Y.-M. para-TsOH-Promoted Cascade Reaction of ortho-Propynol Phenyl Azides for the Synthesis of 4-Methoxy Quinolines and Propargyl Methyl Ethers: Insight on Mechanism of Propargylic Alcohols. Asian J. Org. Chem. 2019, 8, 391–398. [Google Scholar] [CrossRef]
- Li, R.; Jin, F.; Song, X.-R.; Yang, T.; Ding, H.; Yang, R.; Xiao, Q.; Liang, Y.-M. Acid-promoted cyclization of 2-propynolphenols leading to 4-tosyloxy-2H-chromenes. Tetrahedron Lett. 2019, 60, 331–334. [Google Scholar] [CrossRef]
- Li, R.; Song, X.-R.; Chen, X.; Ding, H.; Xiao, Q.; Liang, Y.-M. Copper-Catalyzed Cascade Cyclization of 2-Propynolphenols: Access to 4-Phosphorylated 2H-Chromenes. Adv. Synth. Catal. 2017, 359, 3962–3967. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Ding, H.; Chen, X.; Yang, T.; Jiang, B.; Xiao, Q.; Liang, Y.-M. An efficient approach to 4-chloro quinolines via TMSCl-mediated cascade cyclization of ortho-propynol phenyl azides. Org. Chem. Front. 2018, 5, 1537–1541. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, R.; Yang, T.; Chen, X.; Ding, H.; Xiao, Q.; Liang, Y.-M. Novel and Efficient Access to Flavones under Mild Conditions: Aqueous HI-Mediated Cascade Cyclization/Oxidative Radical Reaction of 2-Propynolphenols. Eur. J. Org. Chem. 2018, 40, 5548–5552. [Google Scholar] [CrossRef]
- Yang, T.; Kou, P.; Jin, F.; Song, X.-R.; Bai, J.; Ding, H.; Xiao, Q.; Liang, Y.-M. TFA-Promoted Sulfonation/Cascade Cyclization of 2-Propynolphenols with Sodium Sulfinates to 4-Sulfonyl 2H-Chromenes under Metal-free Conditions. Org. Chem. Front. 2019, 6, 3162–3166. [Google Scholar] [CrossRef]
- CCDC 1949212 (compound 3a) Contains the Supplementary Cystallographic Data for This Paper. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 26 August 2019).
- Song, X.-R.; Li, R.; Ding, H.; Yang, R.; Xiao, Q.; Liang, Y.-M. Highly efficient access to 4-chloro-2H-chromenes and 1,2-dihydroquinolines under mild conditions: TMSCl-mediated cyclization of 2-propynolphenols/anilines. Tetrahedron Lett. 2016, 57, 4519–4524. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yin, G.; Hong, D.; Lu, P.; Wang, Y. Tandem Reaction of Propargylic Alcohol, Sulfonamide, and N-Iodosuccinimide: Synthesis of N-(2-Iodoinden-1-yl)arenesulfonamide. Org. Lett. 2011, 13, 1024–1027. [Google Scholar] [CrossRef]
Sample Availability: Samples of the final compounds are available from the authors. |






| Entry | Catalyst (x mol%) | Solvent | T [°C] | Yield [%] |
|---|---|---|---|---|
| 1 | Sc(OTf)3 (30) | DCE | 100 | 42 |
| 2 | Zn(OTf)2 (30) | DCE | 100 | 53 |
| 3 | Cu(OTf)2 (30) | DCE | 100 | trace |
| 4 | Cu(OAc)2 (30) | DCE | 100 | trace |
| 5 | CuCl2 (30) | DCE | 100 | <5 |
| 6 | AgOTf (30) | DCE | 100 | trace |
| 7 | Zn(OTf)2 (30) | MeNO2 | 100 | 61 |
| 8 | Zn(OTf)2 (30) | CH3CN | 100 | 81 |
| 9 | Zn(OTf)2 (30) | 1,4-dioxane | 100 | trace |
| 10 | Zn(OTf)2 (30) | DCM | 40 | 32 |
| 11 | Zn(OTf)2 (30) | CH3CN | 80 | 75 |
| 12 | Zn(OTf)2 (30) | CH3CN | 110 | 80 |
| 13 | Zn(OTf)2 (20) | CH3CN | 100 | 82 |
| 14 | Zn(OTf)2 (10) | CH3CN | 100 | 67 |
| 15 b | Zn(OTf)2 (20) | CH3CN | 100 | 71 |
| 16 c | Zn(OTf)2 (20) | CH3CN | 100 | 86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Song, X.-R.; Yang, R.; Ding, H.; Bai, J.; Xiao, Q. An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides. Molecules 2019, 24, 3526. https://doi.org/10.3390/molecules24193526
Yang T, Song X-R, Yang R, Ding H, Bai J, Xiao Q. An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides. Molecules. 2019; 24(19):3526. https://doi.org/10.3390/molecules24193526
Chicago/Turabian StyleYang, Tao, Xian-Rong Song, Ruchun Yang, Haixin Ding, Jiang Bai, and Qiang Xiao. 2019. "An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides" Molecules 24, no. 19: 3526. https://doi.org/10.3390/molecules24193526
APA StyleYang, T., Song, X. -R., Yang, R., Ding, H., Bai, J., & Xiao, Q. (2019). An Efficient Approach to Phosphorylated Isoindoline Fused with Triazoles via Zn-Catalyzed Cascade Cyclization of 2–Propynol Benzyl Azides and Diarylphosphine Oxides. Molecules, 24(19), 3526. https://doi.org/10.3390/molecules24193526