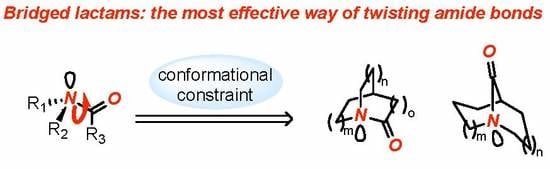
Chemistry of Bridged Lactams: Recent Developments
Abstract
:1. Introduction
2. Synthesis, Properties and Reactivity of Bridged Lactams
3. Bridged Sultams
4. Application in Natural Product Synthesis
5. Miscellaneous Examples
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Pauling, L. The Nature of the Chemical Bond; Oxford University Press: London, UK, 1940. [Google Scholar]
- Szostak, M.; Aubé, J. Chemistry of Bridged Lactams and Related Heterocycles. Chem. Rev. 2013, 113, 5701–5765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szostak, M.; Aubé, J. Medium-Bridged Lactams: A New Class of Non-Planar Amides. Org. Biomol. Chem. 2011, 9, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lease, T.G.; Shea, K.J. A Compilation and Analysis of Structural Data of Distorted Bridgehead Olefins and Amides. In Advances in Theoretically Interesting Molecules; JAI Press Inc.: Greenwich, CT, USA, 1992. [Google Scholar]
- Hall, H.K., Jr.; El-Shekeil, A. Anti-Bredt bridgehead nitrogen compounds in ring-opening polymerization. Chem. Rev. 1983, 83, 549–555. [Google Scholar]
- Poland, B.W.; Xu, M.Q.; Quiocho, F.A. Structural Insights into the Protein Splicing Mechanism of PI-SceI. J. Biol. Chem. 2000, 275, 16408–16413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanelli, A.; Shekhtman, A.; Cowburn, D.; Muir, T.W. Semisynthesis of a segmental isotopically labeled protein splicing precursor: NMR evidence for an unusual peptide bond at the N-extein–intein junction. Proc. Natl. Acad. Sci. USA 2004, 101, 6397–6402. [Google Scholar] [CrossRef] [PubMed]
- Shemella, P.; Pereira, B.; Zhang, Y.M.; Van Roey, P.; Belfort, G.; Garde, S.; Nayak, S.K. Mechanism for Intein C-Terminal Cleavage: A Proposal from Quantum Mechanical Calculations. Biophys. J. 2007, 92, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizak, C.; Gerber, S.; Michaud, G.; Schubert, M.; Fan, Y.Y.; Bucher, M.; Darbare, T.; Aebi, M.; Reymond, J.L.; Locher, K.P. Unexpected reactivity and mechanism of carboxamide activation in bacterial N-linked protein glycosylation. Nat. Commun. 2013, 4, 2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, V.; Holzer, W.; Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Structures of Highly Twisted Amides Relevant to Amide N–C Cross-Coupling: Evidence for Ground-State Amide Destabilization. Chem. Eur. J. 2016, 22, 14494–14498. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.K.; Dunitz, J.D. The non-planar amide group. J. Mol. Biol. 1971, 59, 169–182. [Google Scholar] [CrossRef]
- Szostak, R.; Aubé, J.; Szostak, M. An Efficient Computational Model to Predict Protonation at the Amide Nitrogen and Reactivity along the C–N Rotational Pathway. Chem. Commun. 2015, 51, 6395–6398. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Aubé, J.; Szostak, M. Determination of Structures and Energetics of Small- and Medium-Sized One-Carbon Bridged Twisted Amides using ab Initio Molecular Orbital Methods. Implications for Amidic Resonance along the C–N Rotational Pathway. J. Org. Chem. 2015, 80, 7905–7927. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.; Venanzi, C.A. Structures and energetics of two bridgehead lactams and their N- and O-protonated forms: An ab initio molecular orbital study. J. Am. Chem. Soc. 1993, 115, 6951–6957. [Google Scholar] [CrossRef]
- Greenberg, A.; Moore, D.T.; DuBois, T.D. Small and Medium-Sized Bridgehead Bicyclic Lactams: A Systematic ab Initio Molecular Orbital Study. J. Am. Chem. Soc. 1996, 118, 8658–8668. [Google Scholar] [CrossRef]
- Morgan, J.; Greenberg, A. Novel bridgehead bicyclic lactams: Molecules predicted to have O-protonated and N-protonated tautomers of comparable stability; hyperstable lactams and their O-protonated tautomers. J. Chem. Thermodyn. 2014, 73, 206–212. [Google Scholar] [CrossRef]
- Szostak, R.; Szostak, M. Tröger’s Base Twisted Amides: High Amide Bond Twist and N-/O-Protonation Aptitude. J. Org. Chem. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; VanderVelde, D.G.; Takase, M.K.; Shahgholi, M.; Stoltz, B.M. Total Synthesis and Characterization of 7-Hypoquinuclidonium Tetrafluoroborate and 7-Hypoquinuclidone BF3 Complex. J. Am. Chem. Soc. 2016, 138, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Tani, K.; Stoltz, B.M. Synthesis and structural analysis of 2-quinuclidonium tetrafluoroborate. Nature 2006, 441, 731–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarov, I.V.; Yanik, S.; Ishchenko, A.Y.; Davies, J.E.; Goodman, J.M.; Kirby, A.J. The Most Reactive Amide As a Transition-State Mimic For cis–trans Interconversion. J. Am. Chem. Soc. 2015, 137, 926–930. [Google Scholar] [CrossRef]
- Morgan, K.M.; Rawlins, M.L.; Montgomery, M.N. Influence of methyl substituents on the stability of 1-aza-2-adamantanone, Kirby’s most twisted amide. J. Phys. Org. Chem. 2005, 18, 310–314. [Google Scholar] [CrossRef]
- Kirby, A.J.; Komarov, I.V.; Wothers, P.D.; Feeder, N. The Most Twisted Amide: Structure and Reactions. Angew. Chem. Int. Ed. 1998, 37, 785–786. [Google Scholar] [CrossRef]
- Morgan, J.P.; Weaver-Guevara, H.M.; Fitzgerald, R.W.; Dunlap-Smith, A.; Greenberg, A. Ab initio computational study of 1-methyl-4-silatranone and attempts at its conventional synthesis. Struct. Chem. 2017, 28, 327–331. [Google Scholar] [CrossRef]
- Hu, F.; Lalancette, R.; Szostak, M. Structural Characterization of N-Alkylated Twisted Amides: Consequences for Amide Bond Resonance and N–C Cleavage. Angew. Chem. Int. Ed. 2016, 55, 5062–5066. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Nareddy, P.; Lalancette, R.; Jordan, F.; Szostak, M. σ N−C Bond Difunctionalization in Bridged Twisted Amides: Sew-and-Cut Activation Approach to Functionalized Isoquinolines. Org. Lett. 2017, 19, 2386–2389. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Marsden, S.P.; Nelson, A. Design and synthesis of a fragment set based on twisted bicyclic lactams. Bioorg. Med. Chem. 2018, 26, 3030–3033. [Google Scholar] [CrossRef] [PubMed]
- Steliou, K.; Poupart, M.A. Reagents for organic synthesis. Part 3. Tin-mediated esterification in macrolide synthesis. J. Am. Chem. Soc. 1983, 105, 7130–7138. [Google Scholar] [CrossRef]
- Liniger, M.; Liu, Y.; Stoltz, B. Sequential Ruthenium Catalysis for Olefin Isomerization and Oxidation: Application to the Synthesis of Unusual Amino Acids. J. Am. Chem. Soc. 2017, 139, 13944–13949. [Google Scholar] [CrossRef]
- Brouillette, W.J.; Jestkov, V.P.; Brown, M.L.; Akhtar, M.S.; DeLorey, T.M.; Brown, G.B. Bicyclic Hydantoins with a Bridgehead Nitrogen. Comparison of Anticonvulsant Activities with Binding to the Neuronal Voltage-Dependent Sodium Channel. J. Med. Chem. 1994, 37, 3289–3293. [Google Scholar] [CrossRef]
- Smissman, E.E.; Matuszak, A.J.; Corder, C.N. Reduction of Barbiturates Under Hydroboration Conditions. J. Pharm. Sci. 1964, 53, 1541–1542. [Google Scholar] [CrossRef]
- Szostak, R.; Liu, C.; Lalancette, R.; Szostak, M. Twisted N-Acyl-hydantoins: Rotationally Inverted Urea-Imides of Relevance in N–C(O) Cross-Coupling. J. Org. Chem. 2018, 83, 14676–14682. [Google Scholar] [CrossRef]
- Pereira, R.; Pfeifer, L.; Fournier, J.; Gouverneur, V.; Cvengroš, J. Twisting the ethano-Tröger’s base: The bisamide. Org. Biomol. Chem. 2017, 15, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Artacho, J.; Ascic, E.; Rantanen, T.; Karlsson, J.; Wallentin, C.J.; Wang, R.; Wendt, O.F.; Harmata, M.; Snieckus, V.; Wärnmark, K. Twisted Amide Analogues of Tröger’s Base. Chem. Eur. J. 2012, 18, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, G.; Helmchen, G. Enantioselective Syntheses of Bicyclic Lactams Based on Iridium-Catalyzed Asymmetric Allylic Substitution and Heck Cyclization. Eur. J. Org. Chem. 2014, 2242–2252. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.L.; Wang, F.L.; Guo, Z.; Cheng, Y.F.; Wang, N.; Dong, X.W.; Fang, C.; Liu, J.; Hou, C.; et al. Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings. Nat. Commun. 2016, 7, 13852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drews, J. Drug Discovery: A Historical Perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.A.; Leit, S.M. The First Examples of Bridgehead Bicyclic Sultams. J. Am. Chem. Soc. 1999, 121, 8126–8127. [Google Scholar] [CrossRef]
- Khalifa, A.; Conway, L.; Geoghegan, K.; Evans, P. Ammonium formate-based one-pot reductive Heck reactions for the construction of cyclic sulfonamides. Tetrahedron Lett. 2017, 58, 4559–4562. [Google Scholar] [CrossRef]
- Geoghegan, K.; Smullen, S.; Evans, P. Halonium Ion Triggered Rearrangement of Unsaturated Benzo- Annulated Bi- and Tricyclic Sulfonamides. J. Org. Chem. 2013, 78, 10443–10451. [Google Scholar] [CrossRef]
- Borgohain, H.; Devi, R.; Dheer, D.; Borah, B.J.; Shankar, R.; Das, S.K. Synthesis of Tetrahydroquinoline-Embedded Bridged Benzothiaoxazepine-1,1-dioxides. Eur. J. Org. Chem. 2017, 6671–6679. [Google Scholar] [CrossRef]
- Grosheva, D.S.; Rassadin, V.A.; Sokolov, V.V. A Route to Benzo-Annelated δ-Sultams through Michael Cyclization. Eur. J. Org. Chem. 2015, 2015, 1355–1363. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhu, J. Total Syntheses of (−)-Mersicarpine, (−)-Scholarisine G, (+)-Melodinine E, (−)-Leuconoxine, (−)-Leuconolam, (−)-Leuconodine A, (+)-Leuconodine F, and (−)-Leuconodine C: Self-Induced Diastereomeric Anisochronism (SIDA) Phenomenon for Scholarisine G and Leuconodines A and C. J. Am. Chem. Soc. 2015, 137, 6712–6724. [Google Scholar] [PubMed]
- Yang, Y.; Bai, Y.; Sun, S.; Dai, M. Biosynthetically Inspired Divergent Approach to Monoterpene Indole Alkaloids: Total Synthesis of Mersicarpine, Leuconodines B and D, Leuconoxine, Melodinine E, Leuconolam, and Rhazinilam. Org. Lett. 2014, 16, 6216–6219. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, N.; Banwell, M.G.; Carr, P.D.; Willis, A.C. A Total Synthesis of (±)-3-O-Demethylmacronine through Rearrangement of a Precursor Embodying the Haemanthidine Alkaloid Framework. J. Org. Chem. 2017, 82, 4336–4341. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Mohammed, S.; Robert, F.; Landais, Y. Total Synthesis of (±)-Eucophylline. A Free-Radical Approach to the Synthesis of the Azabicyclo[3.3.1]nonane Skeleton. Org. Lett. 2015, 17, 4518–4521. [Google Scholar] [PubMed]
- Zhang, L.; Wang, Y.; Yao, Z.J.; Wang, S.; Yu, Z.X. Kinetic or Dynamic Control on a Bifurcating Potential Energy Surface? An Experimental and DFT Study of Gold-Catalyzed Ring Expansion and Spirocyclization of 2-Propargyl-β-tetrahydrocarbolines. J. Am. Chem. Soc. 2015, 137, 13290–13300. [Google Scholar] [CrossRef] [PubMed]
- Pfaffenbach, M.; Roller, A.; Gaich, T. Synthesis of Indolophanes by Photochemical Macrocyclization. Chem. Eur. J. 2016, 22, 8444–8447. [Google Scholar] [CrossRef]
- White, C.J.; Hickey, J.L.; Scully, C.C.G.; Yudin, A.K. Site-Specific Integration of Amino Acid Fragments into Cyclic Peptides. J. Am. Chem. Soc. 2014, 136, 3728–3731. [Google Scholar] [CrossRef]
- Zaretsky, S.; Rai, V.; Gish, G.; Forbes, M.W.; Kofler, M.; Yu, J.C.Y.; Tan, J.; Hickey, J.L.; Pawson, T.; Yudin, A.K. Twisted amide electrophiles enable cyclic peptide sequencing. Org. Biomol. Chem. 2015, 13, 7384–7388. [Google Scholar] [CrossRef]
- Chung, B.K.W.; White, C.J.; Scully, C.C.G.; Yudin, A.K. The reactivity and conformational control of cyclic tetrapeptides derived from aziridine-containing amino acids. Chem. Sci. 2016, 7, 6662–6668. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.K. Theoretical insights into structure, bonding, reactivity and importance of ion-pair interactions in Kirby’s tetrafluoroboric acid salts of twisted amides. RSC Adv. 2015, 5, 105668–105677. [Google Scholar] [CrossRef]
- Pandey, K.K. Does hydrohalic acid HX (X = F, Cl) form true N-protonated twisted amide salts? Effects of anions on the ion-pair interactions and on the amide moiety in N-protonated tricyclic twisted amide salts. New J. Chem. 2016, 40, 7831–7839. [Google Scholar] [CrossRef]
- Artamonov, O.S.; Slobodyanyuk, E.Y.; Volochnyuk, D.M.; Komarov, I.V.; Tolmachev, A.A.; Mykhailiuk, P.K. Synthesis of Trifluoromethyl-Substituted 3-Azabicyclo[n.1.0]alkanes: Advanced Building Blocks for Drug Discovery. Eur. J. Org. Chem. 2014, 46, 3592–3598. [Google Scholar] [CrossRef]
- Amatov, T.; Jangra, H.; Pohl, R.; Cisařová, I.; Zipse, H.; Jahn, U. Unique Stereoselective Homolytic C-O Bond Activation in Diketopiperazine-Derived Alkoxyamines by Adjacent Amide Pyramidalization. Chem. Eur. J. 2018, 24, 15336–15345. [Google Scholar] [CrossRef]
- Wang, S.; Taniguchi, T.; Monde, K.; Kawahata, M.; Yamaguchi, K.; Otani, Y.; Ohwada, T. Hydrogen bonding to carbonyl oxygen of nitrogen-pyramidalized amide—Detection of pyramidalization direction preference by vibrational circular dichroism spectroscopy. Chem. Commun. 2016, 52, 4018–4021. [Google Scholar] [CrossRef]
- Mahesh, S.; Tang, K.C.; Raj, M. Amide Bond Activation of Biological Molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Rózsa, B.; Csomos, A.; Csizmadia, I.G.; Mucsi, Z. Amide Activation in Ground and Excited States. Molecules 2018, 23, 2859. [Google Scholar] [CrossRef] [PubMed]
- Glover, S.A.; Rosser, A.A. Heteroatom Substitution at Amide Nitrogen—Resonance Reduction and HERON Reactions of Anomeric Amides. Molecules 2018, 23, 2834. [Google Scholar] [CrossRef]
- Morgan, K.M.; Ashline, D.J.; Morgan, J.P.; Greenberg, A. Electrospray Ionization (ESI) Fragmentations and Dimethyldioxirane Reactivities of Three Diverse Lactams Having Full, Half, and Zero Resonance Energies. J. Org. Chem. 2014, 79, 517–528. [Google Scholar] [CrossRef]
- Glover, S.A.; Rosser, A.A. HERON reactions of anomeric amides: Understanding the driving force. J. Phys. Org. Chem. 2015, 28, 215–222. [Google Scholar] [CrossRef]
- Szostak, R.; Shi, S.; Meng, G.; Lalancette, R.; Szostak, M. Ground-State Distortion in N-Acyl-tert-butyl-carbamates (Boc) and N-Acyl-tosylamides (Ts): Twisted Amides of Relevance to Amide N–C Cross-Coupling. J. Org. Chem. 2016, 81, 8091–8094. [Google Scholar] [CrossRef]
- Meng, G.; Shi, S.; Lalancette, R.; Szostak, R.; Szostak, M. Reversible Twisting of Primary Amides via Ground State N–C(O) Destabilization: Highly Twisted Rotationally Inverted Acyclic Amides. J. Am. Chem. Soc. 2018, 140, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Szostak, R.; Szostak, M. N-Acyl-Glutarimides: Resonance and Proton Affinities of Rotationally-Inverted Twisted Amides Relevant to N−C(O) Cross-Coupling. Org. Lett. 2018, 20, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, S.; Liu, Y.; Liu, R.; Lalancette, R.; Szostak, R.; Szostak, M. The Most Twisted Acyclic Amides: Structures and Reactivity. Org. Lett. 2018, 20, 7771–7774. [Google Scholar] [CrossRef] [PubMed]
























© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szostak, R.; Szostak, M. Chemistry of Bridged Lactams: Recent Developments. Molecules 2019, 24, 274. https://doi.org/10.3390/molecules24020274
Szostak R, Szostak M. Chemistry of Bridged Lactams: Recent Developments. Molecules. 2019; 24(2):274. https://doi.org/10.3390/molecules24020274
Chicago/Turabian StyleSzostak, Roman, and Michal Szostak. 2019. "Chemistry of Bridged Lactams: Recent Developments" Molecules 24, no. 2: 274. https://doi.org/10.3390/molecules24020274
APA StyleSzostak, R., & Szostak, M. (2019). Chemistry of Bridged Lactams: Recent Developments. Molecules, 24(2), 274. https://doi.org/10.3390/molecules24020274