2.1. Germplasm Collection and Identification of Phenolics
The list of
Trifolium species used in this investigation is reported in
Table 1. Both cultivated and natural populations from different environments (lowlands and mountains) were analyzed for their phenolic content. Phenolics occurring in the extract of the 14
Trifolium samples under investigation were separated by UPLC method (see Materials and Methods) and UV spectra were obtained using a photo diode array detector. As an example, the UPLC chromatogram of leaf and flower extracts of
T. repens var.
sylvestre is shown in
Figure 1. Six groups of phenolics were identified based on their characteristic absorption spectra (
Figure 2), namely: phenolic acids (
Figure 2A), clovamides (
Figure 2B), flavanols (
Figure 2C), flavones (
Figure 2D), flavonols (
Figure 2E) and isoflavones (
Figure 2F).
A comparison was made of their retention times and mass spectral data obtained in positive and negative mode with those of standard compounds or with compounds previously reported in literature for
Trifolium spp. [
19,
20,
25,
26,
27,
28]. A tentative identification of phenolics was performed based on key fragment ions and other MS observations. For flavonoids and their glycosyl derivatives the loss of 162
m/z was indicative of hexose (glucose or galactose), the loss of 146
m/z was indicative of rhamnose, the loss of 132
m/z was indicative of pentose (xylose or arabinose). Moreover, the loss of 44
m/z in the negative ion mode and the loss of 86
m/z were indicative of the presence of a malonate.
Branched
C-glycosides were also investigated by the presence of characteristic ions [M − H − 60]
−, [M − H − 90]
−, and [M − H − 120]
− [
25,
26,
27,
28,
29].
Along with clovamide (N-caffeoyl-l-DOPA), phenolic acids were detected in almost all Trifolium extracts in low amount and were mostly constituted by glycosyl derivatives of caffeic acid, ferulic acid and coumaric acid.
Flavanols were identified in very low amount (< 0.3 mg g
−1 dry matter) in some extracts, and identified as catechin/epicatechin and a catechin dimer. Flavones, flavonols and isoflavones represented the main bulk of flavonoid constituents of the extracts. The most abundant, tentatively identified, flavonoids in the 14
Trifolium samples are reported in
Table 2 where their percentage amount in the whole extracts is indicated. All the identified compounds were quantitatively evaluated by appropriate standards (see Experimental).
Glycosyl and glycosyl malonate derivative of luteolin, together with lower amount of derivatives of apigenin and of the isoflavone biochanin A, were the most abundant compounds identified in
T. alexandrinum (#1) extracts. In flowers, large amounts of quercetin galactoside and quercetin glucoside were also detected. All these compounds were previously reported in the species [
30,
31,
32]. High amounts of glycosyl and glycosyl malonate derivative of flavones (quercetin in leaves and quercetin and kaempferol in flowers) and isoflavones (biochanin A and formononetin in leaves and flowers) were detected in red clover (#2 and #7), as previously reported in this widely investigated
Trifolium species [
19,
20,
33,
34,
35,
36]. In snow clover (#3 and #12) extracts, glycosyl and glycosyl malonate derivative of flavones (quercetin) and isoflavones (formononetin and prunetin) were detected in large amounts in both leaves and flowers. The abundant presence of prunetin was reported to be a characteristic feature of this clover species [
20]. Leaf extracts of
T. repens (#4, #8 and #13, see
Figure 1) featured high content of di- and trisaccharide derivative of flavonols quercetin and kaempferol, together with other minor compounds, as already reported [
37,
38,
39,
40]. Flowers were characterized instead by high amount of quercetin galactoside and its acetyl derivative, together with lower content of myricetin galactoside. Subterranean clover (#5 and #6) leaves and flowers confirmed the large presence of isoflavones biochanin A, genistein and formononetin glycosides and glycosyl malonate derivatives [
28].
T. alpinum (#
9) extracts showed a complex mixture of flavonoids. In leaves, they were mainly constituted by mono and diglycosides of quercetin, while kaempferol glycosides were detected in flowers. The presence of glycosides of a quercetin isomer (MW=302) was also detected. The very low amount of isoflavones assessed in this
Trifolium species was inconsistent with previous results [
26]. Di- and tri-saccharide derivatives of quercetin were observed in
T. badium (#10) extracts. An unidentified flavonoid (MW = 462) was abundant in leaves, while a monoglycoside of luteolin/luteolin isomer (MW = 286) was detected in large amount in flowers.
Leaves of
T. ochroleucum (#11) mainly comprised mono- and di-saccharides of quercetin and, to a lesser extent, kaempferol. Quercetin and kaempferol glycosyl malonates were detected as the main constituents of flower extracts. Higher flavonoid content had been previously reported in
T. ochroleucum leaves [
19]. Quercetin and kaempferol diglycosides were abundant in
T. thalii (#14) leaf extract, while the flower extract largely contained myricetin and quercetin glycosides. The isoflavone formononetin glycoside and its malonyl derivative were also detected in leaves.
2.2. Evaluation of Phenolic Compounds in the Trifolium Species in Relation to Their Antioxidant Activities
To facilitate the assessment of the biological activity of the different
Trifolium extracts and to evaluate their antioxidant properties in relation to the phytocomplex (whole extract), the detected compounds were grouped into four distinct classes based on their chemical structure and biological properties, namely, phenolic acids, clovamides, isoflavones, and other flavonoids, this latter including flavanols, flavonols, and flavones. The presence of a fifth group of phenolic compounds, namely proanthocyanidins (or condensed tannins) was assessed by the butanol/HCl method [
41], and evaluated with pelargonidin as a standard. Although with some limitations, this method was reported to allow for the most effective detection of proanthocyanidins [
42,
43,
44]. Pelargonidin was selected as a standard because of its presence in all samples among anthocyanidins obtained from the acid-catalysed cleavage of the condensed tannins. Preliminary investigation performed by a cellulose bidimensional thin layer chromatography (2D TLC) of the obtained anthocyanidin (data not reported), showed the presence of pelargonidin, cyanidin and delfinidin. In all the analyzed samples, variation was observed for these compounds, with pelargonidin being one of the most detected compounds.
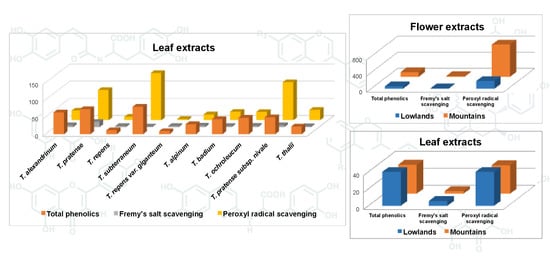
Results of the quantitative evaluation of different phenolics in leaves and flowers of the 14 samples of clover under investigation are reported in
Table 3,
Table 4 and
Table 5.
Flower tissues featured higher concentration of total phenolics compared to leaves across all accessions (
Table 3). However, differences between plant organs for individual phenolic group concentrations were not consistent, with no difference for phenolic acids and clovamides, higher concentration in leaves for isoflavones, and higher concentration in flowers for other flavonoids and for proanthocyanidins. The antioxidant activity was higher in flower than in leaf extracts according to each of the three applied assays.
It is widely accepted that leaves have higher amount of phenolic compounds than flowers, owing to the abundance of pathway precursors in leaves due to photosynthesis [
45,
46]. However, the current result of higher concentration of total phenolics in flowers than in leaves had several precedents in different folk-medicine species, such as yarrow,
Achillea millefolium L. [
47], pomegranate,
Punica granatum L. [
48], or white-weed,
Ageratum conyzoides L. [
49]. The facts that pigments are composed of flavonoids in most flowers, and that proanthocyanidins are produced by closely related branches of the flavonoid pathway using the same metabolic intermediates, make of no surprise the finding that flowers in our germplasm collection were particularly rich in these groups of phenolics. Abeynayake et al. [
50] reported that white clover plants accumulate higher level of proanthocyanidins in flowers than in vegetative tissues. In addition to a possible ultraviolet (UV)-screening effect exerted by phenolics [
22], a role for flavonoids was reported in the development of functional pollen [
51], which may also explain the abundance of these compounds in flower tissues. Total phenolic concentration in flowers, especially as proanthocyanidins, fully supported higher antioxidant activity of flower compared to leaf extracts, no matter the applied scavenging assay. Nonetheless, greater sensitivity of the peroxyl radical scavenging for proanthocyanidins was evident, confirming the results in Jayaprakasha et al. [
52], thus accounting for the current outstanding difference between leaf and flower extracts for this assay.
The 14
Trifolium samples differed significantly (
P < 0.05) for leaf phenolic concentrations and antioxidant activity according to ANOVA (
Table 4). The red clover cultivar Aiace (#2) was rich in phenolic acids, followed by the snow clover population #3 and the berseem clover experimental cultivar #1. Clovamides were found in highest concentration in red clover samples (#2 and #7) and they were also present in the snow clover populations #3 and #12 and the berseem cultivar #1.
The subterranean clover populations #5 and #6 showed the highest concentration of isoflavones, followed by the two red clover samples (#2 and #7) and the two snow clover populations (#3 and #12). Three mountain species, namely, sulphur clover (#11), brown clover (#10) and alpine clover (#9) featured the highest concentration of other flavonoids, together with the berseem clover (#1). The two white clover accessions from lowlands (#4 and #8) and the two subterranean clover populations (#5 and #6) were characterized by the lowest concentration of other flavonoids. Proanthocyanidins were abundant in leaves of brown clover (#10) and berseem clover (#1) only. The leaf concentration of total phenolics exceeded the level of 60 mg g−1 dry matter in the two red clover accessions (#2 and #7), the subterranean clover population #5 and the berseem clover #1, while barely reaching the level of 10 mg g−1 dry matter in the two lowland white clover samples (#8 and #4).
Just as for the leaf samples, there was significant variation among accessions (
P < 0.05) according to ANOVA for the flower phenolic concentrations and antioxidant activity (
Table 5). However, accession patterns of phenolic concentrations were largely different in flowers compared to leaves. Only clovamides showed an outstanding consistency between flowers and leaves of exclusive presence in red clover (#2 and #7), snow clover (#3 and #12) and berseem clover (#1) germplasm (
Table 4 and
Table 5). Concentration of phenolic acids was highest in flowers of the red clover samples (#2 and #7), the snow clover population #3, the sulphur clover population #11 and the berseem clover cultivar #1. Isoflavone concentration (much lower in flowers than in leaves, see
Table 3) was highest in the red clover accessions (#2 and #7), the snow clover population #3 and the subterranean clover population #6. The subterranean clover population #5, featuring the highest isoflavone concentration in leaves, was missing in the flower analysis, owing to too few flowers to be used in the chemical determinations. The brown clover population #10 showed the highest concentration of other flavonoids, followed by the Thal clover population #14, while the subterranean clover population #6 had remarkably low concentration of these compounds. Somewhat similar was the pattern of proanthocyanidin concentration, with highest values in mountain accessions (brown clover #10, Thal clover #14, snow clover #12, and white clover #13) and lowest one in the subterranean clover #6. The very high concentration of proanthocyanidins in the mentioned mountain accessions clearly contributed to their highest concentrations of flower total phenolics, with values exceeding 100 mg/g dry matter, while the subterranean clover population #6 was bottom ranking for flower total phenolics with a concentration of about 10 mg/g dry matter (
Table 5).
Oleszek et al. [
19] partitioned their
Trifolium collection into groups according to the patterns of leaf phenolic composition. The present germplasm also featured accession groups with specific phenolic composition. In some cases, these patterns bore a taxonomic meaning.
T. subterraneum and
T. pratense (the latter, both as red clover and as snow clover subspecies) were characterized by high leaf concentration of isoflavones. Clovamides were restricted to red clover and, to a lesser extent, snow clover and berseem clover. Most alpine species and berseem clover were rich in flavonoids other than isoflavones. White clover (across the three evaluated taxonomic forms) featured the lowest leaf phenolic concentration.
The richness of leaf isoflavones in subterranean clover confirms previous results on this species [
28]. Red clover isoflavone extracts are commercially available as nutraceuticals and they have been proposed as an alternative to hormone-replacement therapy [
53]. Subterranean clover may represent an interesting new source of isoflavones, with higher concentration of these compounds and more diverse pattern of isoflavone composition compared to red clover [
28].
As emphasized [
19], attention should be paid for exploitation to those species comprising good concentration of different phenolic groups, as the presence of these groups may provide synergistic health effects of plant extracts.
Accession rankings for the antioxidant activity of leaf extracts according to the three assays were quite inconsistent (
Table 4). The highest activity was observed in the two red clover samples (#2 and #7) for the Fremy’s salt scavenging, in the subterranean clover population #5, the red clover population #7 and the snow clover population #12 for the peroxyl radical scavenging, and in the brown clover population #10 and the red clover cultivar #2 for the superoxide anion scavenging. The inconsistency of accession ranking for the three assays was confirmed by the lack of significant pairwise correlation between assays based on leaf accession values (data not reported).
Unlike for the data on leaf extracts, there was some consistency of accession ranking between assays for the antioxidant activity of flower extracts (
Table 5). In particular, the Thal clover population #14 and the subterranean clover population #6 were always top- and bottom-ranking, respectively, regardless of the scavenging assay. The mountain white clover population #13 also featured rather high antioxidant activity of flower extract with all three assays. Overall, there were moderately high positive pairwise correlations between scavenging assays, ranging from
r = 0.74,
P < 0.01 (Fremy’s salt vs. peroxyl radical) to
r = 0.87,
P < 0.01 (Fremy’s salt vs. superoxide anion).
The presence of diversified patterns of phytochemical composition, and the contemporary presence of structurally different groups of phenolics, such as isoflavones, other flavonoids and proanthocyanidins, that were likely to determine different responses to the antioxidant assays [
54], justified the choice of three methods of antioxidant measurements. These assays were characterised, indeed, by rather different reactivity towards distinct free radical molecular probes, as successively confirmed by the results of the regression analysis described below.
Lowland accessions had higher concentration of phenolic acids, clovamides and isoflavones, and lower concentration of other flavonoids and proanthocyanidins, than mountain accessions consistently in leaves and flowers. However, lowland germplasm showed higher concentration of total phenolics in leaves, while mountain accessions had higher concentration of total phenolics in flowers (
Table 6). This inconsistency was mostly due to outstanding concentration of isoflavones in leaves of lowland germplasm, and of both proanthocyanidins and flavonoids other than isoflavones in flowers of mountain germplasm. An inconsistency of rankings between germplasm provenances was also largely observed for the antioxidant activity. Lowland accessions had, on average, higher scavenging activity of leaf extracts in two assays out of three, whereas the scavenging activity of flower samples was higher in mountain than in lowland germplasm regardless of the assay (
Table 6).
As already anticipated, the high concentration of flavonoids (other than isoflavones) and proanthocyanidins, associated with higher antioxidant capacities, in flower tissues of mountain germplasm can be a hint of the photoprotection effect recognized to flavonoids and other phenolics [
55], possibly exerted on the sensitive reproductive system [
23]. It is indeed well known that UV levels increase with altitude, and mountain species must be provided with adequate protection. Accumulation of flavonoids in response to UV-B exposition was reported in silver birch,
Betula pendula Roth [
56], while UV-stress-adapted germplasm displayed particularly high constitutive or elicited levels of flavonoids and other phenolics in
Arabidopsis thaliana (L.) Heynh. [
57] and in white clover [
38]. UV absorption is one of the UV-protective properties ascribed to flavonoids, which also include energy dissipation and antioxidant activities [
55]. A role of free-radical scavenging in response to UV exposure can be postulated [
58].
The pattern of phenolic composition in lowland germplasm was suggestive of different adaptive strategies. The abundance of isoflavones in lowland leaves, in particular, may indicate natural selection to decreased palatability for herbivores, or increased defence systems against invertebrate pests [
59].
Table 7 summarizes the best linear models predicted for leaf and flower samples from the multiple regression analysis of the antioxidant activity on mean concentrations of groups of phenolic compounds within the
Trifolium germplasm. The concentration of ‘other flavonoids’ and that of ‘proanthocyanidins’ consistently proved affected by multicollinearity in flowers, and for that reason only the former was retained in the regressions on flower data. Regardless of the scavenging assay and the plant organ, the adjusted
R2 of the models was only moderate at most, with the exception of the good prediction of the Fremy’s salt scavenging in leaves (
R2 = 0.934,
P < 0.001). In both leaves and flowers, the peroxyl radical scavenging had a complex best fitting model, including most of the phenolic groups as regressors, whereas the Fremy’s salt and the superoxide anion scavenging showed simpler models for both plant organ extracts.
The concentration of clovamides had a consistent, positive regression coefficient in the three best models predicted for the antioxidant activity in leaves. Similarly, the concentration of the group of flavonoids other than isoflavones contributed positively to predicting the antioxidant activity with all assays in flowers (
Table 7).
The multicollinearity between the concentrations of flavonoids and proanthocyanidins prevented their simultaneous assessment as regressors in the prediction of the antioxidant activity. However, when taken individually in a simple correlation analysis with the three scavenging assays, the mean flower concentration of proanthocyanidins also proved positively correlated with the antioxidant activity, with correlation ranging between r = 0.63 (P < 0.05) with the peroxyl radical and r = 0.73 (P < 0.01) with the superoxide anion scavenging.
Although claimed as interesting antioxidant compounds, clovamides proved to be important but not critical for the antioxidant activity in cocoa,
Theobroma cacao L. [
60]. Subsequent findings indicated them as potent bioactive compounds with anti-inflammatory activity in human cells [
61]. In the current study, clovamides appeared to have a conditional, positive role in determining the antioxidant activity of leaf extracts in the genus
Trifolium. Our results are in line with those by Kolodziejczyk et al. [
62], who reported that clovamide-rich extract from
T. pallidum reduced the damage induced by oxidative stress to blood platelets and plasma.
A key role in determining the antioxidant activity in flowers of
Trifolium germplasm was exerted instead by the classes of flavonoids (isoflavones excluded) and proanthocyanidins, which was fully justified by the strong antioxidant properties reported for these compounds [
11,
52,
63]. As already mentioned, flower pigmentation and UV screening contribute to the abundance of flavonoids and proanthocyanidins in flower tissues.
This investigation confirmed the genus Trifolium as a great reservoir of phenolic compounds, with different chemical structure and, possibly, largely diversified biological activity. Such a wealth of potentially important metabolites is available for clinical and nutraceutical utilization. It is worth reminding that, unlike other species claimed for extraction of biologically active compounds, most Trifolium species have a well-established agronomic technique for their cultivation. This study also suggested possible links between environmental factors (stresses, in particular) and concentration and composition of phenolic compounds.