Aggregation State of Residual α-Helices and Their Influence on Physical Properties of S. c. ricini Native Fiber
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ordered α-Helix Structure in S. c. ricini Liquid Fibroin As-Cast Film
2.2. Ordered α-Helix Structure in S. c. ricini Native Silk Fiber
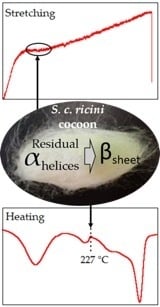
2.3. Impact of Heat-Treatment on the Mechanical Properties of Single S. c. ricini Native Fiber
2.4. Strain-Dependent α-Helix to β-Sheet Transition in S. c. ricini Native Silk Fiber Traced by WAXD
3. Experimental Section
3.1. Preparation of S. c. ricini LFaq As-Cast Film and Native Fiber
3.2. 13C Cross Polarization–Magic Angle Spinning (CP/MAS) Solid-State NMR Spectroscopy
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Wide-Angle X-ray Diffraction Analyses
3.5. Thermal Analyses
3.6. Tensile Tests of Single S. c. ricini Silk Fibers
3.7. Strain-Dependent Structure Transformation Monitored by WAXD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zethner, O.; Koustrup, R.; Nguku, E.; Raina, S.K. African ways of silk, 2nd ed.; Regal Press Kenya Ltd.: Nairobi, Kenya, 2017. [Google Scholar]
- Sezutsu, H.; Yukuhiro, K. The complete nucleotide sequence of the Eri-silkworm (Samia cynthia ricini) fibroin gene. J. Insect Biotechnol. Sericology 2014, 83, 59–70. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, N.M.; Oliveira, M.B.; da Costa, D.P.S.; Naskar, D.; Mano, J.F.; Kundu, S.C.; Reis, R.L. Fabrication and characterization of Eri silk fibers-based sponges for biomedical application. Acta Biomater. 2016, 32, 178–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Talukdar, B.; Bharali, R.; Rajkhowa, R.; Devi, D. Fabrication and characterization of biomaterial film from gland silk of Muga and Eri silkworms. Biopolymers 2013, 99, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Warwicker, J.O. Comparative studies of fibroins: II. The crystal structures of various fibroins. J. Mol. Biol. 1960, 2, 350–362. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Yukuhiro, K.; Kanda, T.; Tamura, T. Preferential codon usage and two types of repetitive motifs in the fibroin gene of the Chinese oak silkworm, Antheraea pernyi. Insect Mol. Biol. 1997, 6, 89–95. [Google Scholar] [CrossRef]
- Sezutsu, H.; Yukuhiro, K. Dynamic rearrangement within the Antheraea pernyi silk fibroin gene is associated with four types of repetitive units. J. Mol. Evol. 2000, 51, 329–338. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, J.; Goo, T.; Yun, E.; Lee, K.; Kim, Y.; Jin, B.; Lee, S.; Kim, K.; Kang, S.; et al. Cloning of the fibroin gene from the oak silkworm, Antheraea yamamai and its complete sequence. Biotechnol. Lett. 2001, 23, 1321–1326. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mita, K.; Arunkumar, K.P.; Nagaraju, J. Molecular architecture of silk fibroin of Indian golden silkmoth, Antheraea assama. Sci. Rep. 2015, 5, 12706. [Google Scholar] [CrossRef]
- Datta, A.; Ghosh, A.K.; Kundu, S.C. Purification and characterization of fibroin from the tropical Saturniid silkworm, Antheraea mylitta. Insect Biochem. Mol. Biol. 2001, 31, 1013–1018. [Google Scholar] [CrossRef]
- Hinman, M.B.; Lewis, V.R. Isolation of a clone encoding a second dragline silk fibroin. J. Biol. Chem. 1992, 267, 19320–19324. [Google Scholar] [PubMed]
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 1999, 202, 3295–3303. [Google Scholar]
- Arnott, S.; Dover, S.D.; Elliott, A. Structure of β-poly-L-alanine: Refined atomic co-ordinates for an anti-parallel beta-pleated sheet. J. Mol. Biol. 1967, 30, 201–208. [Google Scholar] [CrossRef]
- Yang, M.; Yao, J.; Sonoyama, M.; Asakura, T. Spectroscopic characterization of heterogeneous structure of Samia cynthia ricini silk fibroin induced by stretching and molecular dynamics simulation. Macromolecules 2004, 37, 3497–3504. [Google Scholar] [CrossRef]
- Rousseau, M.-E.; Lefevre, T.; Beaulieu, L.; Asakura, T.; Pezolet, M. Study of protein conformation and orientation in silkworm and spider silk fibers using Raman microspectroscopy. Biomacromolecules 2004, 5, 2247–2257. [Google Scholar] [CrossRef]
- Rousseau, M.E.; Beaulieu, L.; Lefèvre, T.; Paradis, J.; Asakura, T.; Pèzolet, M. Characterization by Raman microspectroscopy of the strain-induced conformational transition in fibroin fibers from the silkworm Samia cynthia ricini. Biomacromolecules 2006, 7, 2512–2521. [Google Scholar] [CrossRef]
- Lefèvre, T.; Rousseau, M.-E.; Pézolet, M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Zhang, J.; Jordan, J.S.; Wang, X.; Henning, R.W.; Yarger, J.L. Structural comparison of various silkworm silks: An insight into the structure–property relationship. Biomacromolecules 2018, 19, 906–917. [Google Scholar] [CrossRef]
- van Beek, J.D.; Beaulieu, L.; Schäfer, H.; Demura, M.; Asakura, T.; Meier, B.H. Solid-state NMR determination of the secondary structure of Samia cynthia ricini silk. Nature 2000, 405, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Asakura, T. Structure determination of a peptide model of the repeated helical domain in Samia cynthia ricini silk fibroin before spinning by a combination of advanced solid-state NMR methods. J. Am. Chem. Soc. 2003, 125, 7230–7237. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Nishimura, A.; Kametani, S.; Kawanishi, S.; Aoki, A.; Suzuki, F.; Kaji, H.; Naito, A. Refined crystal structure of Samia cynthia ricini silk fibroin revealed by solid-state NMR investigations. Biomacromolecules 2017, 18, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Gehoh, M.; Yuzuriha, K. Crystal structure of silk (Bombyx mori). J. Polym. Sci. Polym. Phys. 1991, 29, 889–891. [Google Scholar] [CrossRef]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformation characterization of Bombyx mori silk fibroin in the solid state by high-frequency 13C cross polarization-magic angle spinning NMR, X-ray diffraction, and infrared spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawanishi, S.; Yamazaki, T.; Aoki, A.; Saito, H.; Asakura, T. Structural determination of the tandem repeat motif in Samia cynthia ricini liquid silk by solution NMR. Macromolecules 2015, 48, 6574–6579. [Google Scholar] [CrossRef]
- Fang, G.; Sapru, S.; Behera, S.; Yao, J.; Shao, Z.; Kundu, S.C.; Chen, X. Exploration of the tight structural–mechanical relationship in mulberry and non-mulberry silkworm silks. J. Mater. Chem. B 2016, 4, 4337–4347. [Google Scholar] [CrossRef]
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S.; et al. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Structure and physical properties of silkworm cocoons. J. R. Soc. Interface 2012, 9, 2299–2308. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Sumita, I.; Tadokoro, H. Structural studies of polyethers. IX. Planar zigzag modification of poly(ethylene oxide). J. Polym. Sci. Pol. Phys. 1973, 11, 2113–2122. [Google Scholar] [CrossRef]
- Hearle, J.W.S. A critical review of the structural mechanics of wool and hair fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. [Google Scholar] [CrossRef]
- Paquin, R.; Colomban, P. Nanomechanics of single keratin fibres: A Raman study of the α-helix → β-sheet transition and the effect of water. J. Raman Spectrosc. 2007, 38, 504–514. [Google Scholar] [CrossRef]
- Toki, S.; Fujimaki, T.; Okuyama, M. Strain-induced crystallization of natural rubber as detected real-time by wide-angle X-ray diffraction technique. Polymer 2000, 41, 5423–5429. [Google Scholar] [CrossRef]
- Kondo, Y.; Hirabayashi, K.; Iizuka, E.; Go, Y. Studies of the fine structure of silk fibroin: (III). Confirmation of the prescence of helical conformation in Anteraea pernyi silk fibroin. Sen-I Gakkaishi 1967, 23, 311–315. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Ishikawa, H.; Kakudo, M. Changes in the internal fine structure of silk fibroin by drawing. Sen-I Gakkaishi 1968, 25, 440–446. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Bamba, M.; Nishio, S.; Asakura, T. Tightly winding structure of sequential model peptide for repeated helical region in Samia cynthia ricini silk fibroin studied with solid-state NMR. Protein Sci. 2003, 12, 666–671. [Google Scholar] [CrossRef]
- Asakura, T.; Ito, T.; Okudaira, M.; Kameda, T. Structure of alanine and glycine residues of Samia c ynthia ricini silk fibers studied with solid-state 15N and 13C NMR. Macromolecules 1999, 32, 4940–4946. [Google Scholar] [CrossRef]
- Balcytis, A.; Ryu, M.; Wang, X.; Novelli, F.; Seniutinas, G.; Du, S.; Wang, X.; Li, J.; Davis, J.; Appadoo, D.; et al. Silk: Optical properties over 12.6 octaves THz-IR-Visible-UV range. Materials 2017, 10, 356. [Google Scholar] [CrossRef]
- Moseti, K.O.; Yoshioka, T.; Kameda, T.; Nakazawa, Y.; Tokyo University of Agriculture and Technology, Tokyo, Japan. Structure water-solubility relationship in α-helix-rich films cast from aqueous and 1,1,1,3,3,3-hexafluoro-2-propanol solutions of S. c. ricini silk fibroin. Unpublished work. 2019. [Google Scholar]
- Bamford, C.H.; Brown, L.; Elliot, A.; Hanby, W.E.; Trotter, I.F. Alpha and beta-forms of poly-L-alanine. Nature 1954, 173, 27–29. [Google Scholar] [CrossRef]
- Brown, L.; Trotter, I.F. X-ray studies of poly-L-alanine. Trans. Faraday Soc. 1956, 52, 537–548. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Zhang, C.; Lu, S.; Yan, H.; Huang, D.; Ye, H. Study on porous silk fibroin materials. II. Preparation and characteristics of spongy porous silk fibroin materials. J. Appl. Polym. Sci. 2001, 79, 2192–2199. [Google Scholar] [CrossRef]
- Nazarov, R.; Jin, H.; Kaplan, D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Magoshi, J.; Magoshi, Y.; Nakamura, S. Mechanism of fiber formation of silkworm. In Silk polymers, ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tashiro, K.; Ohta, N. Molecular orientation enhancement of silk by the hot-stretching-induced transition from α-helix-HFIP complex to β-sheet. Biomacromolecules 2016, 17, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.L.; Jones, J.A.; Bringhurst, H.N.; Copeland, C.G.; Addison, J.B.; Weber, W.S.; Mou, Q.; Yarger, J.L.; Lewis, R.V. Mechanical and physical properties of recombinant spider silk films using organic and aqueous solvents. Biomacromolecules 2014, 15, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Rajkhowa, R.; Kaur, J.; Wang, X.; Batchelor, W. Intrinsic tensile properties of cocoon silk fibres can be estimated by removing flaws through repeated tensile tests. Interface 2015, 12, 20150177. [Google Scholar] [CrossRef] [Green Version]
- Offord, C.; Vollrath, F.; Holland, C. Environmental effects on the construction and physical properties of Bombyx mori cocoons. J. Mater. Sci. 2016, 51, 10863–10872. [Google Scholar] [CrossRef]
- Hu, X.; Shmlev, K.; Sun, L.; Gil, E.S.; Park, S.H.; Cebe, P.; Kaplan, D.L. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef]
- Guan, J.; Porter, D.; Vollrath, F. Thermally induced changes in dynamic mechanical properties of native silks. Biomacromolecules 2013, 144, 930–937. [Google Scholar] [CrossRef]
- Freddi, G.; Monti, P.; Nagura, M.; Gotoh, Y.; Tsukada, M. Structure and molecular conformation of Tussah silk fibroin films: Effect of heat treatment. J. Polym. Sci. Polym. Phys. 1997, 35, 841–847. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T. X-ray scattering analyses quantitatively revealed periodic hierarchical structure of polyalanineβ-sheet and non-polyalanine amorphous domains in Antheraea assamensis (Muga) silk. J. Silk Sci. Technol. Jpn 2019, 27, 95–101. [Google Scholar] [CrossRef]
Sample Availability: Samples of S. c. ricini LFaq as-cast film and native fiber are available from the authors upon reasonable request. |









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseti, K.O.; Yoshioka, T.; Kameda, T.; Nakazawa, Y. Aggregation State of Residual α-Helices and Their Influence on Physical Properties of S. c. ricini Native Fiber. Molecules 2019, 24, 3741. https://doi.org/10.3390/molecules24203741
Moseti KO, Yoshioka T, Kameda T, Nakazawa Y. Aggregation State of Residual α-Helices and Their Influence on Physical Properties of S. c. ricini Native Fiber. Molecules. 2019; 24(20):3741. https://doi.org/10.3390/molecules24203741
Chicago/Turabian StyleMoseti, Kelvin O., Taiyo Yoshioka, Tsunenori Kameda, and Yasumoto Nakazawa. 2019. "Aggregation State of Residual α-Helices and Their Influence on Physical Properties of S. c. ricini Native Fiber" Molecules 24, no. 20: 3741. https://doi.org/10.3390/molecules24203741
APA StyleMoseti, K. O., Yoshioka, T., Kameda, T., & Nakazawa, Y. (2019). Aggregation State of Residual α-Helices and Their Influence on Physical Properties of S. c. ricini Native Fiber. Molecules, 24(20), 3741. https://doi.org/10.3390/molecules24203741