A Nature-Inspired Design Yields a New Class of Steroids Against Trypanosomatids
Abstract
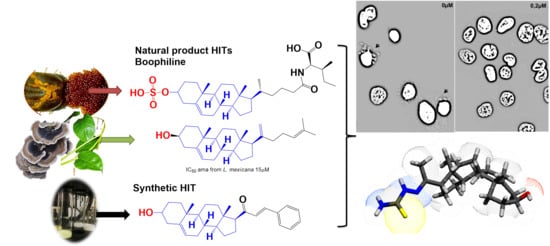
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Steroidal Arylideneketones and Thiazolidenehydrazines
2.2. In Vitro Biological Studies
2.3. Toxicology In Vivo
2.4. Proof of Concept in Vivo
3. Experimental Section
3.1. General
3.1.1. General Synthetic-Procedure for Arylidene Ketones

Compound 5

Compound 3

Compound 2

Compound 4

Compound 1

Compound 15

Compound 6

Compound 7

Compound 16

Compound 17
3.1.2. General Synthetic-Procedure for Thiosemicarbazones

Compound 8

Compound 9

Compound 10
3.1.3. General Synthetic-Procedure for Thiazolylidene Hydrazines

Compound 18

Compound 19

Compound 20

Compound 11
3.1.4. General Procedure for the Preparation of Amides

Compound 14

Compound 12

Compound 13
3.2. Anti-Parasitic Test In Vitro
3.3. Nonspecific In Vitro Cytotoxicity of Mammalian Cells
3.4. Vehicles/Formulation Preparation
3.5. In Vivo Micronucleus Test
3.6. In Vivo Acute Oral Toxicity in Mice
3.7. In Vivo Anti-T. Cruzi Studies (Acute Model)
3.8. In Vivo Anti-Leishmania Studies in Cutaneous Mice Model
3.9. Calculation of the Pharmacokinetic Parameters
3.10. Liver fraction Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esch, K.J.; Petersen, C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013, 26, 58–85. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Mäser, P.; Tadoori, L.P.; Ioset, J.R.; Brun, R.; Sullivan, D.J. Antiprotozoal activity profiling of approved drugs: A starting point toward drug repositioning. PLoS ONE 2015, 10, e0135556. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Salvatella, R.; Issa, J.; Anzola, M.C. A regional fight against Chagas disease: Lessons learned from a successful collaborative partnership. Rev Panam Salud Publica. 2015, 37, 38–43. [Google Scholar] [PubMed]
- WHO. Weekly Epidemiological Record Relevé Épidémiologique Hebdomadaire; World Health Organzation: Geneva, Switzerlamd, 2016; Volume 21, pp. 421–428. [Google Scholar]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chagas Disease (American Trypanosomiasis); online report; World Health Organzation: Geneva, Switzerlamd, 2017. [Google Scholar]
- Dujardin, J.C.; González-Pacanowska, D.; Croft, S.L.; Olesen, O.F.; Späth, G.F. Collaborative actions in anti-trypanosomatid chemotherapy with partners from disease endemic areas. Trends Parasitol. 2010, 26, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Potterat, O.; Hostettmann, K. Boophiline, an Antimicrobial Sterol Amide from the Cattle Tick Boophilus microplus. Helv. Chim. Acta 1997, 80, 2066–2072. [Google Scholar] [CrossRef]
- Pan, L.; Lezama-Davila, C.M.; Isaac-Marquez, A.P.; Calomeni, E.P.; Fuchs, J.R.; Satoskar, A.R.; Kinghorn, A.D. Sterols with antileishmanial activity isolated from the roots of Pentalinon andrieuxii. Phytochemistry 2012, 82, 128–135. [Google Scholar] [CrossRef]
- Leliebre-Lara, V.; Monzote Fidalgo, L.; Pferschy-Wenzig, E.M.; Kunert, O.; Nogueiras Lima, C.; Bauer, R. In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden). Molecules 2016, 21, 1045. [Google Scholar] [CrossRef]
- Lone, I.H.; Khan, K.Z.; Fozdar, B.I.; Hussain, F. Synthesis antimicrobial and antioxidant studies of new oximes of steroidal chalcones. Steroids 2013, 78, 945–950. [Google Scholar] [CrossRef]
- Banday, A.H.; Iqbal Zargar, M.; Ganaie, B. A Synthesis and antimicrobial studies of chalconyl pregnenolones. Steroids 2011, 76, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Shameem, S.A.; Gupta, B.D.; Kumar, H.M.S. D-ring substituted 1,2,3-triazolyl 20-keto pregnenanes as potential anticancer agents: Synthesis and biological evaluation. Steroids 2010, 75, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; El-Sayed, N.N.E.; Abdelaziz, M.A. The Knoevenagel reactions of pregnenolone with cyanomethylene reagents: Synthesis of thiophene, thieno [2,3-b] pyridine, thieno [3,2-d] isoxazole derivatives of pregnenolone and their in vitro cytotoxicity towards tumor and normal cell lines. Steroids 2013, 78, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Al-Omran, F. Reaction of pregnenolone with cyanoacetylhydrazine: Novel synthesis of hydrazide-hydrazone, pyrazole, pyridine, thiazole, thiophene derivatives and their cytotoxicity evaluations. Steroids 2012, 77, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Wardakhan, W.W.; Elmegeed, G.A.; Ashour, R.M.S. Heterocyclizations of pregnenolone: Novel synthesis of thiosemicarbazone, thiophene, thiazole, thieno [2,3-b] pyridine derivatives and their cytotoxicity evaluations. Steroids 2012, 77, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, K.R.; Macedo, A.J.; Giordani, R.B.; Conceição, J.M.; Nicastro, G.G.; Boechat, A.L.; Baldini, R.L.; Abraham, W.R.; Termignoni, C. A steroidal molecule present in the egg wax of the tick Rhipicephalus (Boophilus) microplus inhibits bacterial biofilms. Environ. Microbiol. 2013, 15, 2008–2018. [Google Scholar] [CrossRef]
- Gupta, G.; Peine, K.J.; Abdelhamid, D.; Snider, H.; Shelton, A.B.; Rao, L.; Kotha, S.R.; Huntsman, A.C.; Varikuti, S.; Oghumu, S.; et al. A Novel Sterol Isolated from a Plant Used by Mayan Traditional Healers is Effective in Treatment of Visceral Leishmaniasis Caused by Leishmania donovani. ACS Infect. Dis. 2016, 1, 497–506. [Google Scholar] [CrossRef]
- Vargas-Villavicencio, J.A.; Larralde, C.; Morales-Montor, J. Treatment with dehydroepiandrosterone in vivo and in vitro inhibits reproduction, growth and viability of Taenia crassiceps metacestodes. Int. J. Parasitol. 2008, 38, 775–781. [Google Scholar] [CrossRef]
- Santos, C.D.; Toldo, M.P.A.; Levy, A.M.A.; Kawasse, L.M.; Zucoloto, S.; do Prado, J.C. Dehydroepiandrosterone affects Trypanosoma cruzi tissue parasite burdens in rats. Acta Trop. 2007, 102, 143–150. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Carvalho, P.B.; Avery, M.A.; Tekwani, B.L.; Labadie, G.R. Click chemistry decoration of amino sterols as promising strategy to developed new leishmanicidal drugs. Steroids 2014, 79, 28–36. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Drier-Jonas, S.; Jiménez-Arellanes, M.A. Natural compounds and extracts from Mexican medicinal plants with anti-leishmaniasis activity: An update. Asian Pac. J. Trop. Med. 2017, 10, 1105–1110. [Google Scholar]
- Kakati, D.; Sarma, R.K.; Saikia, R.; Barua, N.C.; Sarma, J.C. Rapid microwave assisted synthesis and antimicrobial bioevaluation of novel steroidal chalcones. Steroids 2013, 78, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.; Ma, P.; Elena, C.; Rivas, A.; Cuchilla, K.; Echeverr, G.; Piro, O.E.; Chorilli, M.; Leal, S.M.; Escobar, P.; et al. Optimization of Antitrypanosomatid Agents: Identification of Nonmutagenic Drug Candidates with in Vivo Activity. J. Med. Chem. 2014, 57, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Varela, J.; Cruces, E.; Fernández, M.; Gabay, M.; Leal, S.M.; Escobar, P.; Sanabria, L.; Serna, E.; Torres, S.; et al. Identification of a New Amide—Containing Thiazole as a Drug Candidate for Treatment of Chagas’ Disease. Antimicrob. Agents Chemother. 2015, 59, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Varela, J.; Birriel, E.; Serna, E.; Torres, S.; Yaluff, G.; DeBilbao, N.V.; Aguirre-López, B.; Cabrera, N.; DíazMazariegos, S.; et al. Potent and Selective Inhibitors of Trypanosoma Cruzi Triosephosphate Isomerase with Concomitant Inhibition of Cruzipain: Inhibition of Parasite Growth through Multitarget Activity. ChemMedChem 2016, 11, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, R.O.; Agüero, F. A simple strain typing assay for Trypanosoma cruzi: Discrimination of major evolutionary lineages from a single amplification product. PLoS Negl. Trop. Dis. 2012, 6, e1777. [Google Scholar] [CrossRef]
- Satragno, D.; Faral-Tello, P.; Canneva, B.; Verger, L.; Lozano, A.; Vitale, E.; Greif, G.; Soto, C.; Robello, C.; Basmadjián, Y. Autochthonous Outbreak and Expansion of Canine Visceral Leishmaniasis, Uruguay. Emerg. Infect. Dis. 2017, 23, 536–538. [Google Scholar] [CrossRef] [Green Version]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef]
- Derendorf, H.; Lesko, L.J.; Chaikin, P.; Colburn, W.A.; Lee, P.; Miller, R.; Powell, R.; Rhodes, G.; Stanski, D.; Venitz, J. Pharmacokinetic/Pharmacodynamic Modeling in Drug Research and Development. J. Clin. Pharmacol. 2000, 40, 1399–1418. [Google Scholar]
- Álvarez, G.; Martínez, J.; Varela, J.; Birriel, E.; Cruces, E.; Gabay, M.; Leal, S.M.; Escobar, P.; Aguirre-lópez, B.; De Gómez-puyou, M.T.; et al. Development of bis-thiazoles as inhibitors of triosephosphate isomerase from Trypanosoma cruzi. Identification of new non-mutagenic agents that are active in vivo. Eur. J. Med. Chem. 2015, 100, 246–256. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Rojas de Arias, A.; Yaluff, G.; De Bilbao, N.V.; Nakayama, H.; Torres, S.; Schinini, A.; Guy, I.; Heinzen, H.; Fournet, A. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine 2010, 17, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Roldos, V.; Nakayama, H.; Rolón, M.; Montero-Torres, A.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; et al. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Perdomo, C.; Coronel, C.; Aguilera, E.; Varela, J.; Aparicio, G.; Zolessi, F.R.; Cabrera, N.; Vega, C.; Rolón, M.; et al. Multi-anti-parasitic activity of arylidene ketones and thiazolidene hydrazines against Trypanosoma cruzi and Leishmania spp. Molecules 2017, 22, 709. [Google Scholar] [CrossRef] [PubMed]
- Schmid, W. The micronucleus test. Mutat. Res. Mutagen. Relat. Subj. 1975, 31, 9–15. [Google Scholar] [CrossRef]
- Fournet, A.; Ferreira, M.E.; Rojas De Arias, A.; Torres De Ortiz, S.; Fuentes, S.; Nakayama, H.; Schinini, A.; Hocquemiller, R. In vivo efficacy of oral and intralesional administration of 2-substituted quinolines in experimental treatment of new world cutaneous leishmaniasis caused by Leishmania amazonensis. Antimicrob. Agents Chemother. 1996, 40, 2447–2451. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Boiani, M.; Merlino, A.; Gerpe, A.; Porcal, W.; Croce, F.; Depaula, S.; Rodríguez, M.A. O-Nitroanilines as major metabolic products of in microsomal and cytosolic fractions of rat hepatocytes and in whole parasitic cells. Xenobiotica 2009, 39, 236–248. [Google Scholar] [CrossRef]
Sample Availability: Samples of the studied compounds are available from the authors. |





| STRUCTURE | No * | IDENTIFIER # | IC50 ± %SD (µM) T. cruzi | IC50 ± %SD (µM) L. amazonensis | IC50 ± %SD (µM) L. infantum | IC50 ± %SD (µM) L. infantum .uy ** |
|---|---|---|---|---|---|---|
| Nifurtimox® | 7 ± 2 | Nd ## | 6 ± 2 | 10±2 | ||
| Glucantime® | Nd | 18 ± 2 | 20 ± 9 | nd | ||
| Miltefosine® | 8 ± 2 | nd | 0.9 ± 0.2 | 5 ± 2 | ||
| Pregnenolone | ˃25 | ˃25 | ˃25 | ˃25 | ||
 | 1 | 1257 | ˃25 | ˃25 | ˃25 | ˃25 |
 | 2 | 1259 | 12 ± 3 | 23 ± 5 | ˃25 | ˃25 |
 | 3 | 1256 | ˃25 | ˃25 | ˃25 | ˃25 |
 | 4 | 1417 | 20 ± 5 | ˃25 | ˃25 | ˃25 |
 | 5 | 1288 | ˃25 | ˃25 | ˃25 | ˃25 |
 | 6 | 1279 | ˃25 | ˃25 | ˃25 | ˃25 |
 | 7 | 1289 | ˃25 | ˃25 | ˃25 | ˃25 |
 | 8 | 1260 | 1.2 ± 0.3 | <22 | 0.2 ± 0.1 | 0.2 ± 0.1 |
 | 9 | 1154 | 8 ± 2 | nd | ˃25 | ˃25 |
 | 10 | 1291 | 25 ± 3 | nd | ˃25 | ˃25 |
 | 11 | 1272 | ˃25 | 16 ± 3 | ˃25 | ˃25 |
 | 12 | 1261 | ˃25 | nd | ˃25 | ˃25 |
 | 13 | 1317 | ˃25 | nd | ˃25 | ˃25 |
 | 14 | 1263 | 8 ± 2 | nd | ˃25 | ˃25 |
| Compound | IC50 ± SD (µM) Murine Macrophages | SI * J774.1/L. amazonensis | SI J774.1/L. infantum | SI J774.1/T. cruzi |
|---|---|---|---|---|
| 2 | 100 ± 10 | 4 | nd | 8 |
| 8 | 50 ± 3 | >2 | 250 | 42 |
| 9 | 25 ± 2 | nd# | nd | 3 |
| 10 | 25 ± 5 | nd | nd | 1 |
| 14 | 100 ± 8 | nd | nd | 13 |
| Glucantime | 15 ± 1 | 1 | 0.5 | nd |
| Miltefosine | 50 ± 7 | nd | 56 | 6 |
| Benznidazole | 400 ± 4 | nd | nd | 57 |
| Treatment * | Number of MnPE ** | Number of PEC + | Media of MnPE ±SD ++ |
|---|---|---|---|
| Control | 19 | 5000 | 4 ± 1 |
| 8 | 24 | 5000 | 5 ± 1 |
| Cyclophosphamide *** (50 mg/kg) | 180 | 5000 | 36 ± 2 |
| Treatment | Doses mg/kg | Doses µmol/kg | Number of Parasites | % of Reduction | % of Survivals |
|---|---|---|---|---|---|
| Chagas | |||||
| Control | 0 | 0 | (0.38 ± 0.02)×106 * | 0 | 60 |
| 8 | 50 | 127 | (0.14 ± 0.03) × 106 * | 62 | 100 |
| Benznidazole | 50 | 200 | (0.01 ± 0.01) × 106 * | 96 | 100 |
| Leishmaniasis | |||||
| Control | 0 | 0 | (29 ± 2) × 106 | 0 | 100 |
| 8 | 50 | 127 | (21 ± 4) × 106 | 27 | 100 |
| Glucantime | 100 | 273 | (25 ± 6) × 106 | 12 | 100 |
| Compound | Solubility (mg/mL) | Gastrointestinal Abortion | Brain Permeability | Skin Penetration (cm/s) | Bioavailability Score | Lipophilicity |
|---|---|---|---|---|---|---|
| Miltefosine | 1.9 × 10−3 | low | No | −4.0 | 0.55 | 3.8 |
| Glucantime | 2.2 × 103 | low | No | −11.3 | 0.55 | −2.9 |
| Benznidazole | 2.3 | high | No | −7.2 | 0.55 | 0.5 |
| 8 | 9.7 × 10−3 | high | No | −5.8 | 0.55 | 3.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera, E.; Perdomo, C.; Espindola, A.; Corvo, I.; Faral-Tello, P.; Robello, C.; Serna, E.; Benítez, F.; Riveros, R.; Torres, S.; et al. A Nature-Inspired Design Yields a New Class of Steroids Against Trypanosomatids. Molecules 2019, 24, 3800. https://doi.org/10.3390/molecules24203800
Aguilera E, Perdomo C, Espindola A, Corvo I, Faral-Tello P, Robello C, Serna E, Benítez F, Riveros R, Torres S, et al. A Nature-Inspired Design Yields a New Class of Steroids Against Trypanosomatids. Molecules. 2019; 24(20):3800. https://doi.org/10.3390/molecules24203800
Chicago/Turabian StyleAguilera, Elena, Cintya Perdomo, Alejandra Espindola, Ileana Corvo, Paula Faral-Tello, Carlos Robello, Elva Serna, Fátima Benítez, Rocío Riveros, Susana Torres, and et al. 2019. "A Nature-Inspired Design Yields a New Class of Steroids Against Trypanosomatids" Molecules 24, no. 20: 3800. https://doi.org/10.3390/molecules24203800
APA StyleAguilera, E., Perdomo, C., Espindola, A., Corvo, I., Faral-Tello, P., Robello, C., Serna, E., Benítez, F., Riveros, R., Torres, S., Vera de Bilbao, N. I., Yaluff, G., & Alvarez, G. (2019). A Nature-Inspired Design Yields a New Class of Steroids Against Trypanosomatids. Molecules, 24(20), 3800. https://doi.org/10.3390/molecules24203800