Annona coriacea Mart. Fractions Promote Cell Cycle Arrest and Inhibit Autophagic Flux in Human Cervical Cancer Cell Lines
Abstract
:1. Introduction
2. Results
2.1. Anonna coriacea Mart. Fractions Contain Acetogenins and Alkaloids in Their Constitution
2.2. A. coriacea Fractions Promote Cytotoxicity in a Dose- and Time-Dependent Manner in Cervical Cancer Cells Lines
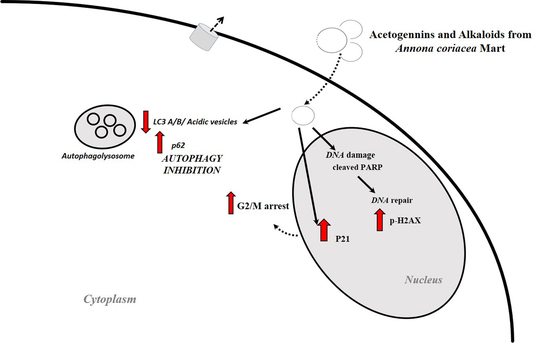
2.3. A. coriacea Fractions Inhibited Cell Proliferation and Invasion, and Induced Cell Cycle Arrest in Cervical Cancer Cell Lines
2.4. Annona coriacea Fractions Promote Cytotoxic Effects by DNA Damage but Do Not Induce Apoptosis
2.5. A. coriacea Fractions Promote Autophagy Flux Inhibition in Cervical Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
4.3. Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (ESI (−) FT-ICR MS)
4.4. Cell Lines and Cell Culture
4.5. Drugs
4.6. Cell Viability and Selectivity Assay
4.7. Proliferation Assay
4.8. Cell Cycle Analysis
4.9. Matrigel Invasion Assay
4.10. Soft Agar Colony Assay
4.11. Annexin-V-7AAD Assay
4.12. Analysis of Autophagy Flux
4.13. Acridine Orange Staining
4.14. Detection of Mitochondrial Membrane Potential
4.15. Comet Assay
4.16. Western Blot
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Goss, P.E.; Lee, B.L.; Badovinac-Crnjevic, T.; Strasser-Weippl, K.; Chávarri-Guerra, Y.; Louis, J.S.; Villarreal-Garza, C.; Unger-Saldaña, K.; Ferreyra, M.; DeBiasi, M.; et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013, 14, 391–436. [Google Scholar] [CrossRef]
- Tota, J.; Ramana–Kumar, A.; El-Khatib, Z.; Franco, E. The road ahead for cervical cancer prevention and control. Curr. Oncol. 2014, 21, e255–e264. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the Cervix Uteri. Int. J. Gynecol. Obstet. 2006, 95, S43–S103. [Google Scholar] [CrossRef]
- Chen, J.; Solomides, C.; Parekh, H.; Simpkins, F.; Simpkins, H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 1217–1227. [Google Scholar] [CrossRef]
- Martinho, O.; Silva-Oliveira, R.; Cury, F.P.; Barbosa, A.M.; Granja, S.; Evangelista, A.F.; Marques, F.; Miranda-Gonçalves, V.; Cardoso-Carneiro, D.; De Paula, F.E.; et al. HER Family Receptors are Important Theranostic Biomarkers for Cervical Cancer: Blocking Glucose Metabolism Enhances the Therapeutic Effect of HER Inhibitors. Theranostics 2017, 7, 717–732. [Google Scholar] [CrossRef] [Green Version]
- Orfanelli, T.; Jeong, J.M.; Doulaveris, G.; Holcomb, K.; Witkin, S.S. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int. J. Cancer 2014, 135, 519–528. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef]
- Peng, X.; Gong, F.; Chen, Y.; Jiang, Y.; Liu, J.; Yu, M.; Zhang, S.; Wang, M.; Xiao, G.; Liao, H. Autophagy promotes paclitaxel resistance of cervical cancer cells: Involvement of Warburg effect activated hypoxia-induced factor 1-alpha-mediated signaling. Cell Death Dis. 2014, 5, e1367. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Chen, X.; Liang, Y.; Yang, D.; Dong, J.; Yang, N.; Liang, Z. Angelicin inhibits the malignant behaviours of human cervical cancer potentially via inhibiting autophagy. Exp. Ther. Med. 2019, 18, 3365–3374. [Google Scholar] [CrossRef] [Green Version]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.A.O.; Alves, A.L.V.; Rosa, M.N.; Silva, L.R.V.; Melendez, M.E.; Cury, F.P.; Gomes, I.N.F.; Tansini, A.; Longato, G.B.; Martinho, O.; et al. Hexane partition from Annona crassiflora Mart. promotes cytotoxity and apoptosis on human cervical cancer cell lines. Investig. New Drugs 2019, 37, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Wan, W.; Zhang, H. In vitro mitochondria-mediated anticancer and antiproliferative effects of Annona glabra leaf extract against human leukemia cells. J. Photochem. Photobiol. B Boil. 2018, 189, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, A.; Figadere, B.; Zafra-Polo, M.C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Kaplan, M.A.C. Propriedades Analgésica e Antiinflamatória do Extrato Metanólico de Folhas de Annona coriacea Mart. (Annonaceae). Lat. Am. J. Pharm. 2007, 26, 872–877. [Google Scholar]
- Formagio, A.; Vieira, M.; Volobuff, C.; Silva, M.; Matos, A.; Cardoso, C.; Foglio, M.; Carvalho, J. In vitro biological screening of the anticholinesterase and antiproliferative activities of medicinal plants belonging to Annonaceae. Braz. J. Med. Boil. Res. 2015, 48, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Ma, C.; Wang, Q.; Shi, Y.; Li, Y.; Wang, X.; Li, X.; Chen, Y.; Chen, J. Three new antitumor annonaceous acetogenins from the seeds of Annona squamosa. Nat. Prod. Res. 2017, 31, 2085–2090. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias Júnior, H.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.G.; Li, T.M.; Sun, L.; Luo, X.Z.; Ding, W.; Li, D.Y. [Studies on the chemical constituents of the seeds from Artabostrys hexapetalus (Annonaceae)]. Yao Xue Xue Bao Acta Pharm. Sin. 2001, 36, 281–286. [Google Scholar]
- Pinheiro, M.L.B.; Xavier, C.M.; De Souza, A.D.L.; Rabelo, D.D.M.; Batista, C.L.; Batista, R.L.; Campos, F.R.; Barison, A.; Valdez, R.H.; Ueda-Nakamura, T.; et al. Acanthoic acid and other constituents from the stem of Annona amazonica (Annonaceae). J. Braz. Chem. Soc. 2009, 20, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-S.; Zeng, L.; Alali, F.; Rogers, L.L.; Wu, F.-E.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Mono-Tetrahydrofuran Ring Acetogenins, Annomuricin E and Muricapentocin, from the Leaves of Annona muricata. J. Nat. Prod. 1998, 61, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; He, K.; Oberlies, N.H.; Zeng, L.; Shi, G.; Evert, D.; McLaughlin, J.L. Longimicins A−D: Novel Bioactive Acetogenins fromAsimina longifolia(Annonaceae) and Structure−Activity Relationships of Asimicin Type of Annonaceous Acetogenins. J. Med. Chem. 1996, 39, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wu, F.-E.; McLaughlin, J.L. Annohexocin, a novel mono-THF acetogenin with six hydroxyls, from Annona muricata (Annonaceae). Bioorg. Med. Chem. Lett. 1995, 5, 1865–1868. [Google Scholar] [CrossRef]
- Morré, D.J.; De Cabo, R.; Farley, C.; Oberlies, N.H.; McLaughlin, J.L. Mode of action of bullatacin, a potent antitumor acetogenin: Inhibition of NADH oxidase activity of HeLa and HL-60, but not liver, plasma membranes. Life Sci. 1995, 56, 343–348. [Google Scholar] [CrossRef]
- Kim, G.S.; Zeng, L.; Alali, F.; Rogers, L.L.; Wu, F.E.; Sastrodihardjo, S.; McLaughlin, J.L. Muricoreacin and murihexocin C, mono-tetrahydrofuran acetogenins, from the leaves of Annona muricata. Phytochemistry 1998, 49, 565–571. [Google Scholar] [CrossRef]
- Alali, F.Q.; Rogers, L.; Zhang, Y.; McLaughlin, J.L. Goniotriocin and (2,4-cis- and -trans)-xylomaticinones, bioactive annonaceous acetogenins from Goniothalamus giganteus. J. Nat. Prod. 1999, 62, 31–34. [Google Scholar] [CrossRef]
- Hui, Y.-H.; Rupprecht, J.K.; Anderson, J.E.; Wood, K.V.; McLaughlin, J.L. Bullatalicinone, a new potent bioactive acetogenin, and squamocin from annona bullata (Annonaceae). Phytother. Res. 1991, 5, 124–129. [Google Scholar] [CrossRef]
- Vamanu, E. Antioxidant Properties of Mushroom Mycelia Obtained by Batch Cultivation and Tocopherol Content Affected by Extraction Procedures. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, E.F.; Roblot, F.; Laprevote, O.; Paulo Mde, Q.; Hocquemiller, R. Two unusual acetogenins from the roots of Annona salzmanii. J. Nat. Prod. 2003, 66, 755–758. [Google Scholar] [CrossRef]
- Da Silva, E.L.M.; Roblot, F.; Hocquemiller, R.; Serani, L.; Laprevote, O. Structure elucidation of annoheptocins, two new heptahydroxylated C37 acetogenins by high-energy collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1936–1944. [Google Scholar] [CrossRef]
- Costa, M.S.; Cossolin, J.F.S.; Pereira, M.J.B.; Sant’Ana, A.E.G.; Lima, M.D.; Zanuncio, J.C.; Serrão, J.E. Larvicidal and Cytotoxic Potential of Squamocin on the Midgut of Aedes aegypti (Diptera: Culicidae). Toxins 2014, 6, 1169–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamkhande, P.G.; Wattamwar, A.S. Annona reticulata Linn. (Bullock’s heart): Plant profile, phytochemistry and pharmacological properties. J. Tradit. Complement. Med. 2015, 5, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, Y.; Ma, C.; Wang, X.; Li, Y.; Miao, Y.; Chen, J.; Li, X. Antitumor activity of Annona squamosa seed oil. J. Ethnopharmacol. 2016, 193, 362–367. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, N.; Cautain, B.; Melguizo, A.; Cortes, D.; Vicente, F.; Genilloud, O.; Tormo, J.R.; Peláez, F. Analysis of cytotoxic activity at short incubation times reveals profound differences among Annonaceus acetogenins, inhibitors of mitochondrial Complex I. J. Bioenerg. Biomembr. 2013, 45, 145–152. [Google Scholar] [CrossRef]
- Yu, J.G.; Gui, H.Q.; Luo, X.Z.; Sun, L. Murihexol, a linear acetogenin from Annona muricata. Phytochemistry 1998, 49, 1689–1692. [Google Scholar] [CrossRef]
- Da Silva, E.L.M.; Roblot, F.; Mahuteau, J.; Cavé, A. Coriadienin, the First Annonaceous Acetogenin with Two Double Bonds Isolated fromAnnona coriaceae. J. Nat. Prod. 1996, 59, 528–530. [Google Scholar] [CrossRef]
- Qin, G.-W.; Li, C.-J.; Wang, L.-Q.; Li, Y.; Min, B.-S.; Nakamura, N.; Hattori, M. Cytotoxic Mono-Tetrahydrofuran Ring Acetogenins from Leaves of Annona montana. Planta Medica 2001, 67, 847–852. [Google Scholar]
- Zhong, J.; Ying, C.; Ruo-Yun, C.; De-Quan, Y. Linear acetogenins from Goniothalamus donnaiensis. Phytochemistry 1998, 49, 769–775. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chou, C.-J.; Wu, Y.-C.; Chang, F.-R.; Liaw, C.-C.; Chiu, H.-F. New Adjacent Bis-Tetrahydrofuran Annonaceous Acetogenins from Annona muricata. Planta Medica 2003, 69, 241–246. [Google Scholar]
- Liaw, C.-C.; Chang, F.-R.; Lin, C.-Y.; Chou, C.-J.; Chiu, H.-F.; Wu, M.-J.; Wu, Y.-C. New cytotoxic monotetrahydrofuran annonaceous acetogenins from Annona muricata. J. Nat. Prod. 2002, 65, 470–475. [Google Scholar] [CrossRef]
- Da Silva, E.L.M.; Roblot, F.; Laprévote, O.; Sérani, L.; Cavé, A. Coriaheptocins A and B, the First Heptahydroxylated Acetogenins, Isolated from the Roots of Annona coriacea. J. Nat. Prod. 1997, 60, 162–167. [Google Scholar] [CrossRef]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a new aporphine alkaloid from the leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Lin, M.; Wang, Y.; Lai, Y.; Hu, J.; Fu, T.; Wang, L.; Lin, S.; Chen, L.; Guo, Y. Curcumin induces the apoptosis of A549 cells via oxidative stress and MAPK signaling pathways. Int. J. Mol. Med. 2015, 36, 1118–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Yao, Z.-J.; Wang, L.-S. Effect of annonaceous acetogenin mimic AA005 on proliferative inhibition of leukemia cells in vitro and its possible mechanisms. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2012, 20, 549–553. [Google Scholar] [PubMed]
- Nordin, N.; Majid, N.A.; Hashim, N.M.; Rahman, M.A.; Hassan, Z.; Ali, H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des. Dev. Ther. 2015, 9, 1437–1448. [Google Scholar]
- Hung, D.T.; Jamison, T.F.; Schreiber, S.L. Understanding and controlling the cell cycle with natural products. Chem. Boil. 1996, 3, 623–639. [Google Scholar] [CrossRef] [Green Version]
- Kielbik, M.; Krzyżanowski, D.; Pawlik, B.; Klink, M. Cisplatin-induced ERK1/2 activity promotes G1 to S phase progression which leads to chemoresistance of ovarian cancer cells. Oncotarget 2018, 9, 19847–19860. [Google Scholar] [CrossRef]
- Chen, K.; Shou, L.-M.; Lin, F.; Duan, W.-M.; Wu, M.-Y.; Xie, X.; Xie, Y.-F.; Li, W.; Tao, M. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anti-Cancer Drugs 2014, 25, 652–662. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Broustas, C.G.; Lieberman, H.B. DNA Damage Response Genes and the Development of Cancer Metastasis. Radiat. Res. 2014, 181, 111–130. [Google Scholar] [CrossRef]
- Zaman, M.S.; Chauhan, N.; Yallapu, M.M.; Gara, R.K.; Maher, D.M.; Kumari, S.; Sikander, M.; Khan, S.; Zafar, N.; Jaggi, M.; et al. Curcumin Nanoformulation for Cervical Cancer Treatment. Sci. Rep. 2016, 6, 20051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; DeSelm, C.; Helms, C.; Bowcock, A.; Rogers, B.E.; Rader, J.; Grigsby, P.W.; Schwarz, J.K. AKT Inhibitors Promote Cell Death in Cervical Cancer through Disruption of mTOR Signaling and Glucose Uptake. PLoS ONE 2014, 9, e92948. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Liu, S.; Zhao, Q.; Liang, X.; Ma, Z.; Gupta, P.K.; Zhao, M.; Wang, A. Autophagy flux inhibition, G2/M cell cycle arrest and apoptosis induction by ubenimex in glioma cell lines. Oncotarget 2017, 8, 107730–107743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliano, A.P.; Pimentel, E.F.; Buzina, A.R.; Vieira, T.Z.; Romão, W.; Tose, L.V.; Lenz, D.; de Andrade, T.U.; Fronza, M.; Kondratyuk, T.P.; et al. Brown seaweed Padina gymnospora is a prominent natural wound-care product. Rev. Bras. Farmacogn. 2016, 26, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Silva-Oliveira, R.J.; Silva, V.A.O.; Martinho, O.; Cruvinel-Carloni, A.; Melendez, M.E.; Rosa, M.N.; De Paula, F.E.; Viana, L.D.S.; Carvalho, A.L.; Reis, R.M. Cytotoxicity of allitinib, an irreversible anti-EGFR agent, in a large panel of human cancer-derived cell lines: KRAS mutation status as a predictive biomarker. Cell. Oncol. 2016, 39, 253–263. [Google Scholar] [CrossRef]
- Mead, T.J.; Lefebvre, V. Proliferation assays (BrdU and EdU) on skeletal tissue sections. Breast Cancer 2014, 1130, 233–243. [Google Scholar]
- Visagie, M.H.; Joubert, A.M. In vitro effects of 2-methoxyestradiol-bis-sulphamate on reactive oxygen species and possible apoptosis induction in a breast adenocarcinoma cell line. Cancer Cell Int. 2011, 11, 43. [Google Scholar] [CrossRef]
- Silva-Oliveira, R.J.; Melendez, M.; Martinho, O.; Zanon, M.F.; Viana, L.D.S.; Carvalho, A.L.; Reis, R.M. AKT can modulate the in vitro response of HNSCC cells to irreversible EGFR inhibitors. Oncotarget 2017, 8, 53288–53301. [Google Scholar] [CrossRef]
- Geissmann, Q. OpenCFU, a New Free and Open-Source Software to Count Cell Colonies and Other Circular Objects. PLoS ONE 2013, 8, 54072. [Google Scholar] [CrossRef]
- Wilson, E.N.; Bristol, M.L.; Di, X.; Maltese, W.A.; Koterba, K.; Beckman, M.J.; Gewirtz, D.A. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm. Cancer 2011, 2, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.T.; Barbosa, M.C.D.S.; De Camargos, L.F.; Da Silva, I.V.G.; Varotti, F.D.P.; Da Silva, L.M.; Moreira, L.M.; Lyon, J.P.; Santos, V.J.D.S.V.D.; Dos Santos, F.V. Digoxin reduces the mutagenic effects of Mitomycin C in human and rodent cell lines. Cytotechnology 2017, 69, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |






| Measured m/z | Theoretical m/z | Error (ppm) | DBE | [M-H]− | Proposed Compound | Reference |
|---|---|---|---|---|---|---|
| 255.2332 | 255.23324 | −1.12 | 1 | [C16H32O2–H+]− | palmitic acid | Chen et al., 2016 |
| 281.24881 | 281.24886 | 0.92 | 2 | [C18H34O2–H+]− | oleic acid | Chen et al., 2016 |
| 595.45815 | 595.45822 | −0.49 | 4 | [C35H64O7–H+]− | asitrocinone | Adewole e Ojewole et al., 2008 |
| 595.45838 | 595.45845 | −0.87 | 4 | [C35H64O7–H+]− | annonacin | Alkofahi et al., 1988 |
| 609.43885 | 609.43893 | −2.85 | 5 | [C35H62O8–H+]− | trilobalicin | He et al., 1997 |
| 611.45312 | 611.45314 | −0.49 | 4 | [C35H64O8–H+]− | annomuricin E | Kim et al., 1998 |
| 621.4742 | 621.4743 | −1.17 | 5 | [C37H66O7–H+]− | asimicin | Ye et al., 1996 |
| 621.47413 | 621.47418 | −0.96 | 5 | [C37H66O7–H+]− | bullatacin | Morre et al., 1995 |
| 627.4483 | 627.44832 | −0.9 | 4 | [C35H64O9–H+]− | annohexocin | Moghadamtousi et al., 2015 |
| 627.44823 | 627.44828 | −0.83 | 4 | [C35H64O9–H+]− | murihexocin | Kim et al., 1998 |
| 635.4540 | 635.4542 | −2.14 | 6 | [C37H64O8–H+]− | goniotriocin | Alali et al., 1999 |
| 637.46921 | 637.46927 | −1.22 | 5 | [C37H66O8–H+]− | bullatalicinone | Hui et al., 1991 |
| 637.46905 | 637.46914 | −1.02 | 5 | [C37H66O8–H+]− | annoglaucin | Bermejo et al., 2005 |
| 641.42889 | 641.42895 | −1.34 | 4 | [C35H64O10–H+]− | coriaheptocin B/A | Formagio et al., 2015 |
| 651.44943 | 651.44949 | −2.65 | 6 | [C35H64O10–H+]− | ginsenoside Rh5 | Vamanu, 2014 |
| 653.46442 | 653.46444 | −1.58 | 5 | [C37H66O9–H+]− | salzmanolin | Queiroz et al., 2003 |
| 669.46005 | 669.4601 | −1.22 | 6 | [C37H68O10–H+]− | annoheptocin A | Meneses Da Silva et al., 1998 |
| 671.47569 | 671.47575 | −1 | 6 | [C37H68O10–H+]− | annoheptocin B | Meneses Da Silva et al., 1998 |
| 763.47932 | 763.47939 | −0.83 | 12 | [C39H70O5–H]− | squamocin glycosilated | Jamkhande e Wattamwar, 2015 |
| IC50 Value (Mean ± SD) µg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | C1 | C2 | C3 | C4 | C5 | C6 | C7 | Cisplatin |
| CaSki | 17.8 ± 2.8 | ND | 6.5 ± 1.8 | ND | 3.6 ± 0.9 | 11.7 ± 2.2 | 21.4 ± 3.3 | 1.05 ± 1.2 |
| HeLa | 12.2 ± 1.5 | ND | 6.6 ± 1.2 | ND | 4.1 ± 0.4 | 12.9 ± 1.9 | 12.3 ± 0.83 | 13.6 ± 0.44 |
| SiHa | 16.1 ± 2.7 | ND | 8.7 ± 1.3 | ND | 5.1 ± 0.6 | 12.6 ± 1.6 | 12.7 ± 1.3 | 15.5 ± 0.93 |
| IC50 Value (Mean ± SD) µg/mL and SI ª | ||||||
|---|---|---|---|---|---|---|
| Cell Line | C3 | C5 | Cisplatin | SIC3 | SIC5 | SI Cisplatin |
| CaSki | 6.5 ± 1.8 | 3.6 ± 0.9 | 1.05 ± 1.2 | 1.57 | 3.72 | 4.57 |
| HeLa | 6.6 ± 1.2 | 4.1 ± 0.4 | 13.6 ± 0.44 | 1.55 | 3.27 | 0.35 |
| SiHa | 8.7 ± 1.3 | 5.1 ± 0.6 | 15.5 ±0.93 | 1.17 | 2.63 | 0.31 |
| HaCat | 10.2 ± 2.4 | 13.4 ± 1.0 | 4.8 ± 1.3 | R | R | R |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, I.N.F.; Silva-Oliveira, R.J.; Oliveira Silva, V.A.; Rosa, M.N.; Vital, P.S.; Barbosa, M.C.S.; dos Santos, F.V.; Junqueira, J.G.M.; Severino, V.G.P.; Oliveira, B.G.; et al. Annona coriacea Mart. Fractions Promote Cell Cycle Arrest and Inhibit Autophagic Flux in Human Cervical Cancer Cell Lines. Molecules 2019, 24, 3963. https://doi.org/10.3390/molecules24213963
Gomes INF, Silva-Oliveira RJ, Oliveira Silva VA, Rosa MN, Vital PS, Barbosa MCS, dos Santos FV, Junqueira JGM, Severino VGP, Oliveira BG, et al. Annona coriacea Mart. Fractions Promote Cell Cycle Arrest and Inhibit Autophagic Flux in Human Cervical Cancer Cell Lines. Molecules. 2019; 24(21):3963. https://doi.org/10.3390/molecules24213963
Chicago/Turabian StyleGomes, Izabela N. Faria, Renato J. Silva-Oliveira, Viviane A. Oliveira Silva, Marcela N. Rosa, Patrik S. Vital, Maria Cristina S. Barbosa, Fábio Vieira dos Santos, João Gabriel M. Junqueira, Vanessa G. P. Severino, Bruno G Oliveira, and et al. 2019. "Annona coriacea Mart. Fractions Promote Cell Cycle Arrest and Inhibit Autophagic Flux in Human Cervical Cancer Cell Lines" Molecules 24, no. 21: 3963. https://doi.org/10.3390/molecules24213963
APA StyleGomes, I. N. F., Silva-Oliveira, R. J., Oliveira Silva, V. A., Rosa, M. N., Vital, P. S., Barbosa, M. C. S., dos Santos, F. V., Junqueira, J. G. M., Severino, V. G. P., Oliveira, B. G., Romão, W., Reis, R. M., & Ribeiro, R. I. M. d. A. (2019). Annona coriacea Mart. Fractions Promote Cell Cycle Arrest and Inhibit Autophagic Flux in Human Cervical Cancer Cell Lines. Molecules, 24(21), 3963. https://doi.org/10.3390/molecules24213963