Lasianosides A–E: New Iridoid Glucosides from the Leaves of Lasianthus verticillatus (Lour.) Merr. and Their Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Spectroscopic Analyses of the Compounds
2.1.1. Chemical Structure of Lasianoside A
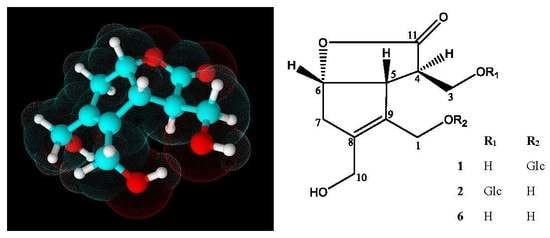
2.1.2. Chemical Structure of Lasianoside B
2.1.3. Chemical Structure of Lasianoside C
2.1.4. Chemical Structure of Lasianoside D
2.1.5. Chemical Structure of Lasianoside E
2.2. Antioxidant and Cytotoxic Activities of Isolated Compounds
3. Materials and Methods
3.1. General Method
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of Compounds 1–5
3.5. Acid Hydrolysis
3.6. Alkaline Hydrolysis
3.7. DPPH Radical Scavenging Activity
3.8. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta BBA Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Robbrecht, E. Tropical Woody Rubiaceae. In Opera Botanica Belgica; National Botanic Garden of Belgium: Meies, Belgium, 1988; Volume 1, p. 132. [Google Scholar]
- Zhu, H. Two new subspecies of Lasianthus inodorus (Rubiaceae) from Kinabalu, Borneo, and their biogeographical implication. Bumea 2001, 46, 447–455. [Google Scholar]
- Wiart, C. Medicinal Plants of the Asia-Pacific—Drugs for the Future; World Scientific Pub Co. Pte Lt.: Singapore, 2006; p. 588. [Google Scholar]
- Napiroon, T.; Balslev, H.; Duangjai, S.; Sookchaloem, D.; Chayamarit, K.; Santimaleeworagun, W.; Vajrodaya, S. Antibacterial property testing of two species of tropical plant lasianthus (rubiaceae). Southeast Asian J. Trop. Med. Public Health 2017, 48, 117–123. [Google Scholar] [PubMed]
- Li, B.; Zhang, D.M.; Luo, Y.M. A new sesquiterpene lactone from the roots of Lasianthus acuminatissimus. Acta Pharm. Sin. 2006, 41, 426–430. [Google Scholar]
- Puff, C.; Chamchumroon, V. Non–indigenous Rubiaceae grown in Thailand. Thai. For. Bull. (Bot.) 2003, 31, 75–94. [Google Scholar]
- Yang, D.; Zhang, C.; Liu, X.X.; Wang, K.; Cheng, Z.Q. Chemical Constituents and Antioxidant Activity of Lasianthus hartii. Chem. Nat. Compd. 2017, 53, 390–393. [Google Scholar] [CrossRef]
- Takeda, Y.; Shimidzu, H.; Mizuno, K.; Inouchi, S.; Masuda, T.; Hirata, E.; Shinzato, T.; Aramoto, M.; Otsuka, H. An Iridoid Glucoside Dimer and a Non-glucosidic Iridoid from the Leaves of Lasianthus wallichii. Chem. Pharm. Bull. 2002, 50, 1395–1397. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.M.; Luo, Y.M.; Chen, X.G.; Zhang, D.; Luo, Y.; Chen, X. Three New and Antitumor Anthraquinone Glycosides from Lasianthus acuminatissimus Merr. Chem. Pharm. Bull. 2006, 37, 297–300. [Google Scholar] [CrossRef]
- Li, B.; Lai, X.W.; Xu, X.H.; Yu, B.W.; Zhu, Y. A new anthraquinone from the root of Lasianthus acuminatissimus. Acta Pharm. Sin. 2007, 42, 502–504. [Google Scholar]
- Li, B.; Zhang, D.M.; Luo, Y.M. Chemical constituents from root of Lasianthus acuminatissimus I. China J. Chin. Mater. Med. 2006, 31, 133–135. [Google Scholar]
- Dallavalle, S.; Jayasinghe, L.; Kumarihamy, B.M.M.; Merlini, L.; Musso, L.; Scaglioni, L. A New 3, 4-seco-Lupane Derivative from Lasianthus gardneri. J. Nat. Prod. 2004, 67, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Roos, M.C.; Ridsdale, C.E. A taxonomic revision of the Malasian species of Lasianthus (Rubiaceae). Blumea 2012, 57, 1–102. [Google Scholar] [CrossRef]
- Napiroon, T.; Tanruean, K.; Vajrodaya, S. Comparative phytochemical evaluation and biological control properties from Lasianthus verticillatus (Lour.) Merr. (Rubiaceae) extracts. Res. J. Biotechnol. 2019, 14, 41–49. [Google Scholar]
- Demirezer, L.O.; Gurbuz, F.; Guvenlap, Z.; Stroch, K.; Zeeck, A. Iridoid, flavonoids and monoterpene glycosides from Galium verum subsp. Verum. Turk. J. Chem. 2006, 30, 525–534. [Google Scholar]
- Masek, A. Determination of Antioxidant Activity of Caffeic Acid and p-Coumaric Acid by Using Electrochemical and Spectrophotometric Assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Moon, J.; Terano, J. Antioxidant activity of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotine. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Sugimoto, S.; Wanas, A.S.; Mizuta, T.; Matsunami, K.; Kamel, M.S.; Otsuka, H. Structure elucidation of secondary metabolites isolated from the leaves of Lxora undulate and their inhibitory activity toward advanced glycation end-products formation. Phytochemistry 2014, 108, 189–195. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sugimoto, S.; Nakamura, S.; Matsuda, H. Medicinal flowers. XXII. Structures of chakasaponins V and VI, chakanoside I, and chakaflavonoside a from flower buds of Chinese tea plant (Camellia sinensis). Chem. Pharm. Bull. 2008, 56, 1297–1303. [Google Scholar] [CrossRef]
- Matsunami, K.; Takamori, I.; Shinzato, T.; Aramoto, M.; Kondo, K.; Otsuka, K.; Takeda, Y. Radical-scavenging activities of new megastimane glucosides from Macaranga tanarius (L.) MULL-ARG. Chem. Pharm. Bull. 2006, 54, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.N.; Sugimoto, S.; Matsunami, K.; Otsuka, H.; Kamel, M.S. One new flavonoid xyloside and one new natural triterpene rhamnoside from the leaves of Syzygium grande. Phytochem. Lett. 2014, 10, 86–90. [Google Scholar] [CrossRef]
- Korkina, L.; Kostyuk, V.; Potapovich, A.; Mayer, W.; Talib, N.; De Luca, C. Secondary Plant Metabolites for Sun Protective Cosmetics: From Pre-Selection to Product Formulation. Cosmetics 2018, 5, 32. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The Unusual Reaction of Semiquinone Radicals with Molecular Oxygen. J. Org. Chem. 2008, 73, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 4.28 br d (12.0) 4.61 br d (12.0) | 4.16 br d (13.0) 4.33 br d (13.0) | 4.16 br d (13.0) 4.27 br d (13.0) | 4.75 br s | 4.18 br d (13.4) 4.28 br d (13.4) |
| 3 | 3.91 2H d (3.9) | 3.93 dd (9.7, 5.5) 4.23 dd (9.7, 4.0) | 4.02 dd (10.0, 5.0) 4.13 m | 4.04 dd (10.1, 3.5) 4.09 dd (10.1, 4.4) | 4.04 dd (10.0, 5.0) 4.13 dd (10.0, 3.4) |
| 4 | 2.99 m | 3.00 m | 2.98 m | 2.94 m | 2.99 m |
| 5 | 3.73 br d (6.3) | 3.78 br d (6.3) | 3.75 m | 3.68 br d (6.3) | 3.76 m |
| 6 | 5.14 t (6.3) | 5.21 td (6.3, 1.0) | 5.16 t (6.4) | 5.13 t (6.3) | 5.15 t (6.4) |
| 7 | 2.75 br d (18.1) 2.95 dd (18.1, 6.3) | 2.73 br d (18.1) 2.94 dd (18.1, 6.3) | 2.69 br d (18.0) 2.85 dd (18.0, 6.4) | 2.69 br d (18.3) 2.82 dd (18.3, 6.3) | 2.62 br d (18.0) 2.82 dd (18.0, 6.4) |
| 8 | - | - | - | - | - |
| 9 | - | - | - | - | - |
| 10 | 4.20 br d (13.4) 4.24 br d (13.4) | 4.20 2H, br s | 4.13 2H, br s | 4.11 br d (13.7) 4.19 br d (13.7) | 4.67 2H, br s |
| 11 | - | - | - | - | - |
| 12 | - | - | |||
| 13 | 2.04 3H, s | 2.03 3H, s | |||
| 1′ | 4.30 d (7.9) | 4.33 d (7.9) | 4.37 d (8.0) | 4.36 d (8.0) | 4.63 d (7.9) |
| 2′ | 3.18 dd (9, 7.9) | 3.21 dd (9.1, 7.9) | 3.23 t (8.0) | 3.24 dd (9.1, 8.0) | 3.23 dd (9.0, 7.9) |
| 3′ | 3.37 t (8.8) | 3.38 t (9.1) | 3.40 m | 3.40 m | 3.40 m |
| 4′ | 3.28 m | 3.30 m | 3.39 m | 3.39 m | 3.39 m |
| 5′ | 3.29 m | 3.31 m | 3.56 m | 3.56 m | 3.56 m |
| 6′ | 3.68 dd (12, 5.5) 3.90 dd (12, 1.8) | 3.72 dd (12, 5.2) 3.89 dd (12, 1.3) | 4.34 dd (12.0, 5.7) 4.57 dd (12.0, 1.8) | 4.43 dd (12.0, 5.7) 4.59 dd (12.0, 2.0) | 4.35 dd (11.9, 6.0) 4.56 dd (11.9, 2.0) |
| 1″ | - | - | - | ||
| 2″ | 7.07 d (1.7) | 7.05 d (2.0) | 7.06 d (2.0) | ||
| 3″ | - | - | - | ||
| 4″ | - | - | - | ||
| 5″ | 6.80 d (8.2) | 6.80 d (8.2) | 6.80 d (8.2) | ||
| 6″ | 6.98 dd (8.2, 1.7) | 6.97 dd (8.2, 2.0) | 6.97 dd (8.2, 2.0) | ||
| 7″ | 7.57 br d (15.9) | 7.56 br d (15.9) | 7.56 br d (15.9) | ||
| 8″ | 6.30 br d (15.9) | 6.28 br d (15.9) | 6.30 br d (15.9) | ||
| 9″ | - | - | - |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 64.4 | 56.8 | 58.4 | 59.4 | 57.1 |
| 3 | 63.3 | 70.5 | 71.2 | 71.2 | 71.3 |
| 4 | 48.4 | 46.8 | 46.9 | 47.0 | 46.8 |
| 5 | 53.4 | 53.0 | 53.4 | 53.4 | 53.3 |
| 6 | 83.2 | 83.3 | 83.4 | 83.3 | 83.2 |
| 7 | 41.8 | 41.7 | 41.6 | 41.5 | 41.7 |
| 8 | 140.2 | 138.5 | 138.5 | 142.0 | 133.4 |
| 9 | 134.4 | 137.4 | 137.4 | 132.5 | 140.5 |
| 10 | 58.4 | 58.4 | 57.0 | 58.5 | 60.9 |
| 11 | 180.9 | 180.2 | 180.2 | 180.0 | 180.0 |
| 12 | 172.6 | 172.6 | |||
| 13 | 20.8 | 20.7 | |||
| 1′ | 103.8 | 104.6 | 104.8 | 104.8 | 104.9 |
| 2′ | 75.1 | 75.0 | 75.0 | 75.0 | 75.0 |
| 3′ | 78.0 | 77.9 | 77.7 | 77.8 | 77.7 |
| 4′ | 71.6 | 71.5 | 71.6 | 71.6 | 71.6 |
| 5′ | 78.0 | 78.1 | 75.5 | 75.5 | 75.5 |
| 6′ | 62.8 | 62.7 | 64.3 | 64.2 | 64.3 |
| 1″ | 127.6 | 127.6 | 127.6 | ||
| 2″ | 115.2 | 115.2 | 115.2 | ||
| 3″ | 146.8 | 146.9 | 146.9 | ||
| 4″ | 149.7 | 149.8 | 149.7 | ||
| 5″ | 116.5 | 116.5 | 116.5 | ||
| 6″ | 123.1 | 123.1 | 123.1 | ||
| 7″ | 147.3 | 147.3 | 147.3 | ||
| 8″ | 114.8 | 114.8 | 114.8 | ||
| 9″ | 169.1 | 169.0 | 169.1 |
| Isolated Compounds | DPPH (IC50 µM) | A549 Cytotoxicity (IC50 µM) |
|---|---|---|
| Lasianoside A (1) | >100 | >100 |
| Lasianoside B (2) | >100 | >100 |
| Lasianoside C (3) | 30.2 ± 1.8 | >100 |
| Lasianoside D (4) | 32.0 ± 1.2 | >100 |
| Lasianoside E (5) | 46.4 ± 2.3 | >100 |
| Lasianol (6) | >100 | >100 |
| Deacetyl daphylloside (7) | >100 | >100 |
| Daphylloside (8) | >100 | >100 |
| Trolox | 29.2 ± 0.39 | - |
| Doxorubicin | - | 0.90 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamoud, G.A.; Saud Orfali, R.; Perveen, S.; Mizuno, K.; Takeda, Y.; Nehira, T.; Masuda, K.; Sugimoto, S.; Yamano, Y.; Otsuka, H.; et al. Lasianosides A–E: New Iridoid Glucosides from the Leaves of Lasianthus verticillatus (Lour.) Merr. and Their Antioxidant Activity. Molecules 2019, 24, 3995. https://doi.org/10.3390/molecules24213995
Al-Hamoud GA, Saud Orfali R, Perveen S, Mizuno K, Takeda Y, Nehira T, Masuda K, Sugimoto S, Yamano Y, Otsuka H, et al. Lasianosides A–E: New Iridoid Glucosides from the Leaves of Lasianthus verticillatus (Lour.) Merr. and Their Antioxidant Activity. Molecules. 2019; 24(21):3995. https://doi.org/10.3390/molecules24213995
Chicago/Turabian StyleAl-Hamoud, Gadah Abdulaziz, Raha Saud Orfali, Shagufta Perveen, Kenta Mizuno, Yoshio Takeda, Tatsuo Nehira, Kazuma Masuda, Sachiko Sugimoto, Yoshi Yamano, Hideaki Otsuka, and et al. 2019. "Lasianosides A–E: New Iridoid Glucosides from the Leaves of Lasianthus verticillatus (Lour.) Merr. and Their Antioxidant Activity" Molecules 24, no. 21: 3995. https://doi.org/10.3390/molecules24213995
APA StyleAl-Hamoud, G. A., Saud Orfali, R., Perveen, S., Mizuno, K., Takeda, Y., Nehira, T., Masuda, K., Sugimoto, S., Yamano, Y., Otsuka, H., & Matsunami, K. (2019). Lasianosides A–E: New Iridoid Glucosides from the Leaves of Lasianthus verticillatus (Lour.) Merr. and Their Antioxidant Activity. Molecules, 24(21), 3995. https://doi.org/10.3390/molecules24213995