A Ratiometric and Colorimetric Hemicyanine Fluorescent Probe for Detection of SO2 Derivatives and Its Applications in Bioimaging
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-Vis and Fluorescence Spectra for Sulphite Detection
2.2. The Selective Response of Probe to Sulfite
2.3. Time-Dependence and pH-Dependence in the Detection Process of Sulfite
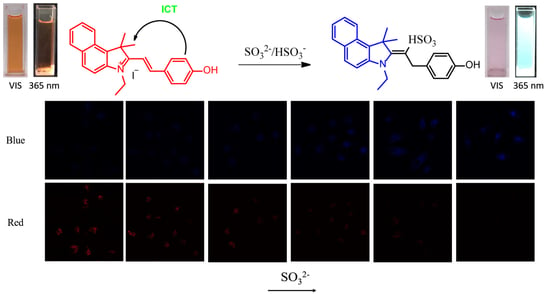
2.4. Reaction Mechanism
2.5. Sulfite Detection in Real Samples
2.6. Fluorescence Imaging in Living Cells
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Preparation of Probe EB
3.3. Preparation of Solutions and Spectra Measurements
3.4. Measurements of Sulfite in Real Samples
3.5. Methods for Fluorescent Quantum Yield
3.6. Limit of Detection
3.7. Cell Culture and Fluorescence Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meng, Z.; Qin, G.; Zhang, B.; Bai, J. DNA damaging effects of sulfur dioxide derivatives in cells from various organs of mice. Mutagenesis 2004, 19, 465–468. [Google Scholar] [CrossRef]
- Shi, X. Generation of SO3- and OH radicals in SO32− reactions with inorganic environmental pollutants and its implications to SO32− toxicity. J. Inorg. Biochem. 1994, 56, 155–165. [Google Scholar] [CrossRef]
- Yin, C.X.; Li, X.Q.; Yue, Y.K.; Chao, J.B.; Zhang, Y.B.; Huo, F.J. A new fluorescent material and its application in sulfite and bisulfite bioimaging. Sens. Actuat. B-Chem. 2017, 246, 615–622. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, Z.; Li, J.; Zhang, Q. The vasorelaxant effect and its mechanisms of sodium bisulfite as a sulfur dioxide donor. Chemosphere 2012, 89, 579–584. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, W.; Liu, X.J.; Kuang, Y.Q.; Jiang, J.H. A novel mitochondria-targeted near-infrared fluorescence probe for ultrafast and ratiometric detection of SO2 derivatives in live cells. Talanta 2017, 168, 203–209. [Google Scholar] [CrossRef]
- Iwasawa, S.; Kikuchi, Y.; Nishiwaki, Y.; Nakano, M.; Michikawa, T.; Tsuboi, T.; Tanaka, S.; Uemura, T.; Ishigami, A.; Nakashima, H.; et al. Effects of SO2 on respiratory system of adult Miyakejima resident 2 years after returning to the island. J. Occup. Health 2009, 51, 38–47. [Google Scholar] [CrossRef]
- Chen, T.M.; Gokhale, J.; Shofer, S.; Kuschner, W.G. Outdoor air pollution: Nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef]
- Schneider, M.; Turke, A.; Fischer, W.J.; Kilmartin, P.A. Determination of the wine preservative sulphur dioxide with cyclic voltammetry using inkjet printed electrodes. Food Chem. 2014, 159, 428–432. [Google Scholar] [CrossRef]
- Grings, M.; Moura, A.P.; Parmeggiani, B.; Pletsch, J.T.; Cardoso, G.M.F.; August, P.M.; Matte, C.; Wyse, A.T.S.; Wajner, M.; Leipnitz, G. Bezafibrate prevents mitochondrial dysfunction, antioxidant system disturbance, glial reactivity and neuronal damage induced by sulfite administration in striatum of rats: Implications for a possible therapeutic strategy for sulfite oxidase deficiency. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017, 1863, 2135–2148. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Q.; Yang, D.; Zhang, H.; Song, X.; Foley, J. Selective, Highly Sensitive Fluorescent Probe for the Detection of Sulfur Dioxide Derivatives in Aqueous and Biological Environments. Anal. Chem. 2014, 87, 609–616. [Google Scholar] [CrossRef]
- Xiang, K.; Chang, S.; Feng, J.; Li, C.; Ming, W.; Liu, Z.; Liu, Y.; Tian, B.; Zhang, J. A colorimetric and ratiometric fluorescence probe for rapid detection of SO2 derivatives bisulfite and sulfite. Dye. Pigment. 2016, 134, 190–197. [Google Scholar] [CrossRef]
- Rajantie, H.; Williams, D.E. Electrochemical titrations of thiosulfate, sulfite, dichromate and permanganate using dual microband electrodes. Analyst 2001, 126, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Türke, A.; Fischer, W.J.; Beaumont, N.; Kilmartin, P.A. Electrochemistry of sulfur dioxide, polyphenols and ascorbic acid at poly(3,4-ethylenedioxythiophene) modified electrodes. Electrochim. Acta. 2012, 60, 184–192. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Hinkley, J.T.; Donne, S.W.; Lindquist, S.E. The electrochemical oxidation of aqueous sulfur dioxide: A critical review of work with respect to the hybrid sulfur cycle. Electrochim. Acta. 2010, 55, 573–591. [Google Scholar] [CrossRef]
- Sullivan, J.; Douek, M. Analysis of hydroxide, inorganic sulphur species and organic anions in kraft pulping liquors by capillary electrophoresis. J. Chromatogr. A 2004, 1039, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Ito, K.; Hirokado, M.; Uematsu, Y.; Suzuki, K.; Suzuki, S.; Saito, K. Determination of sulfur dioxide content of grape skin extract and elderberry color by capillary electrophoresis. Shokuhin. Eiseigaku. Zasshi 2000, 41, 144–148. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, J.B.; Zhang, J.; Chen, B.C.; Kan, J.F.; Zhang, W.F.; Zhou, J.; Ma, H.M. A red lysosome-targeted fluorescent probe for carboxylesterase detection and bioimaging. J. Mater. Chem. B 2019, 7, 2989–2996. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.; Fan, S.; Duan, L.; Li, R. Ratiometric fluorescent probe for rapid detection of bisulfite through 1,4-addition reaction in aqueous solution. J. Agric. Food Chem. 2014, 320, 62–3405. [Google Scholar] [CrossRef]
- Tan, L.; Lin, W.; Zhu, S.; Yuan, L.; Zheng, K. A coumarin-quinolinium-based fluorescent probe for ratiometric sensing of sulfite in living cells. Org. Biomol. Chem. 2014, 12, 4637–4643. [Google Scholar] [CrossRef]
- Li, D.P.; Wang, Z.Y.; Cui, J.; Wang, X.; Miao, J.Y.; Zhao, B.X. A new fluorescent probe for colorimetric and ratiometric detection of sulfur dioxide derivatives in liver cancer cells. Sci. Rep.-UK 2017, 7, 45294–45301. [Google Scholar]
- Niu, T.T.; Yu, T.; Yin, G.X.; Chen, H.M.; Yin, P.; Li, H.T. A novel colorimetric and ratiometric fluorescent probe for sensing SO2 derivatives and their bio-imaging in living cells. Analyst 2019, 144, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qi, H.; Yang, X.F. A ratiometric fluorescent probe for bisulphite anion, employing intramolecular charge transfer. Luminescence 2013, 28, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, W.; Zhai, Q.; Feng, G. Selenocysteine detection and bioimaging in living cells by a colorimetric and near-infrared fluorescent turn-on probe with a large stokes shift. Biosens. Bioelectron. 2017, 87, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yin, G.; Niu, T.; Yin, P.; Li, H.; Zhang, Y.; Chen, H.; Zeng, Y.; Yao, S. A novel colorimetric and fluorescent probe for simultaneous detection of SO32−/HSO3− and HSO4− by different emission channels and its bioimaging in living cells. Talanta 2018, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, W.; Qian, J. A ratiometric fluorescence probe for selective detection of sulfite and its application in realistic samples. Talanta 2017, 162, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, Z.; Feng, G. Colorimetric and ratiometric fluorescent detection of bisulfite by a new HBT-hemicyanine hybrid. Anal. Chim. Acta. 2016, 920, 72–79. [Google Scholar] [CrossRef]
- Xu, W.; Teoh, C.L.; Peng, J.; Su, D.; Yuan, L.; Chang, Y.T. A mitochondria-targeted ratiometric fluorescent probe to monitor endogenously generated sulfur dioxide derivatives in living cells. Biomaterials 2015, 56, 1–9. [Google Scholar] [CrossRef]
- Lan, J.S.; Zeng, R.F.; Ding, Y.; Zhang, Y.; Zhang, T.; Wu, T. A simple pyrene–hemicyanine fluorescent probe for colorimetric and ratiometric detection of SO2 derivatives in the mitochondria of living cells and zebrafish in vivo. Sens. Actuators B Chem. 2018, 268, 328–337. [Google Scholar] [CrossRef]
- Li, C.; Plamont, M.A.; Aujard, I.; Le Saux, T.; Jullien, L.; Gautier, A. Design and characterization of red fluorogenic push-pull chromophores holding great potential for bioimaging and biosensing. Org. Biomol. Chem. 2016, 14, 9253–9261. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Yu, H.; Gao, X.; Shao, S. Mitochondria-Targeted Fluorescent Probe for Imaging Hydrogen Peroxide in Living Cells. Anal. Chem. 2016, 88, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Liu, Y.; Niu, J.; Ni, Z.; Lin, W. A new pyrene-based fluorescent probe with large Stokes shift for detecting hydrogen peroxide in aqueous solution and living cells. J. Photochem. Photobiol. A Chem. 2017, 348, 1–7. [Google Scholar] [CrossRef]
- Kumar, A.; Pandith, A.; Kim, H.-S. Pyrene-appended imidazolium probe for 2,4,6-trinitrophenol in water. Sens. Actuators B Chem. 2016, 231, 293–301. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, J.; Sun, Z.; Fu, B.; Qin, C.; Zeng, L.; Hu, X. A twisted intramolecular charge transfer probe for rapid and specific detection of trace biological SO2 derivatives and bio-imaging applications. Chem. Commun. 2015, 51, 1154–1156. [Google Scholar] [CrossRef]
Sample Availability: Not available. |








| Sample a | SO32− Level Found (μM) | Added (μM) | Found (μM) | Recovery (%) |
|---|---|---|---|---|
| Tap water | 0 b | 25.0 | 24.99 ± 0.55 | 99.9 |
| 0 c | 50.0 | 48.87 ± 0.73 | 97.7 | |
| 100.0 | 95.92 ± 3.70 | 95.9 | ||
| River water | 1.8 ± 0.09 b | 25.0 | 25.9 ± 0.96 | 96.6 |
| 1.5 ± 0.18 c | 50.0 | 52.4 ± 2.53 | 101.2 | |
| 100.0 | 102.3 ± 1.94 | 100.5 | ||
| Sugar | 0 b | 25.0 | 25.1 ± 1.31 | 100.4 |
| 0 c | 50.0 | 52.9 ± 0.55 | 105.8 | |
| 100.0 | 104.8 ± 1.72 | 104.8 | ||
| Chrysanthemum | 16.8 ± 0.18 b | 25.0 | 43.73 ± 2.38 | 107.7 |
| 15.9 ± 0.25 c | 50.0 | 69.2 ± 3.26 | 104.8 | |
| 100.0 | 115.9 ± 2.93 | 99.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.-H.; Jiang, X.-Y.; Que, Y.-F.; Gu, J.-Y.; Wu, T.; Aihemaiti, A.; Shi, K.-X.; Kang, W.-Y.; Hu, B.-Y.; Lan, J.-S.; et al. A Ratiometric and Colorimetric Hemicyanine Fluorescent Probe for Detection of SO2 Derivatives and Its Applications in Bioimaging. Molecules 2019, 24, 4011. https://doi.org/10.3390/molecules24214011
Qin Y-H, Jiang X-Y, Que Y-F, Gu J-Y, Wu T, Aihemaiti A, Shi K-X, Kang W-Y, Hu B-Y, Lan J-S, et al. A Ratiometric and Colorimetric Hemicyanine Fluorescent Probe for Detection of SO2 Derivatives and Its Applications in Bioimaging. Molecules. 2019; 24(21):4011. https://doi.org/10.3390/molecules24214011
Chicago/Turabian StyleQin, Yan-Hong, Xiao-Yi Jiang, Yuan-Fang Que, Jing-Yi Gu, Tong Wu, Ayinazhaer Aihemaiti, Ke-Xin Shi, Wen-Yu Kang, Bi-Ying Hu, Jin-Shuai Lan, and et al. 2019. "A Ratiometric and Colorimetric Hemicyanine Fluorescent Probe for Detection of SO2 Derivatives and Its Applications in Bioimaging" Molecules 24, no. 21: 4011. https://doi.org/10.3390/molecules24214011
APA StyleQin, Y. -H., Jiang, X. -Y., Que, Y. -F., Gu, J. -Y., Wu, T., Aihemaiti, A., Shi, K. -X., Kang, W. -Y., Hu, B. -Y., Lan, J. -S., Ding, Y., & Zhang, T. (2019). A Ratiometric and Colorimetric Hemicyanine Fluorescent Probe for Detection of SO2 Derivatives and Its Applications in Bioimaging. Molecules, 24(21), 4011. https://doi.org/10.3390/molecules24214011