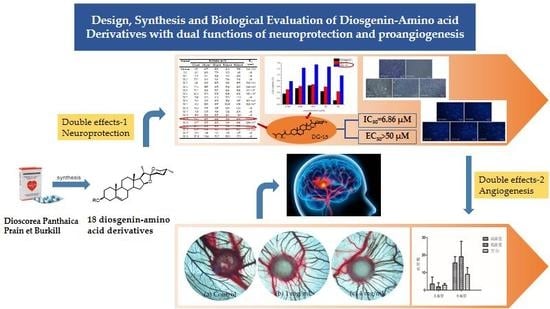
Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis
Abstract
:1. Introduction
2. Results
2.1. Chemical Synthesis
2.2. Biological Activities
2.2.1. Neuroprotective Activity Test Using SH-HY5Y Cell
2.2.2. Biosafety Evaluation Using H9c2 Cell
2.2.3. Morphological Observation Using Giemsa and DAPI Staining
2.2.4. Promotes Angiogenesis in CAM Assay
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Chemical Syntheses
4.2.1. The procedure for esterification at C3-OH (method 1)
4.2.2. The procedure for deprotection reaction (method 2)
4.3. Bio-Evaluation Methods
4.3.1. Cell Culture
4.3.2. Neuroprotective Activity Test Using SH-SY5Y Cell
4.3.3. Biosafety Evaluation Using H9c2 Cell
4.3.4. Morphological Analysis Using Giemsa and DAPI Staining
4.3.5. Neuroprotective Activity Test Using CAM model
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Xu, T.Y.; Guan, Y.F.; Tian, W.W.; Viollet, B.; Rui, Y.C.; Miao, C.Y. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate–activated kinase pathway. Ann. Neurol. 2011, 69, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, X.; Xian, L.; Guo, Z.; Ito, Y.; Sun, W. Potential neuroprotection of protodioscin against cerebral ischemia-reperfusion injury in rats through intervening inflammation and apoptosis. Steroids 2016, 113, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tang, X.N.; Yenari, M.A. The inflammatory response in stroke. J. Neuroimmunol. 2007, 184, 53–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuel, G.E.; Johnson, T.; Liu, D. Therapeutic angiogenesis of exosomes for ischemic stroke. Int. J. Physiol. Pathophysiol. Pharmacol. 2017, 9, 188. [Google Scholar]
- Selvamani, A.; Sathyan, P.; Miranda, R.C.; Sohrabji, F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS ONE 2012, 7, e32662. [Google Scholar] [CrossRef]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef]
- Sautour, M.; Mitaine-Offer, A.C.; Lacaille-Dubois, M.A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, X.; Zhao, J.; Qian, C.; Guo, Z.; Ito, Y.; Sun, W. Diosgenin attenuates the brain injury induced by transient focal cerebral ischemia-reperfusion in rats. Steroids 2016, 113, 103–112. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Yang, B.; Jiang, F.; Zhao, J.; Ouyang, Z. Neuroprotective effects of diosgenin in rats with experimental spinal cord injury via promotion of autophagy. Int. J. Clin. Exp. Med. 2017, 10, 11655–11663. [Google Scholar]
- Zhang, X.; Jin, M.; Tadesse, N.; Dang, J.; Zhou, T.; Zhang, H.; Ito, Y. Dioscorea zingiberensis CH Wright: An overview on its traditional use, phytochemistry, pharmacology, clinical applications, quality control and toxicity. J. Ethnopharmacol. 2018, 220, 283–293. [Google Scholar] [CrossRef]
- Jesus, M.; Martins, A.P.; Gallardo, E.; Silvestre, S. Diosgenin: Recent highlights on pharmacology and analytical methodology. J. Anal. Methods Chem. 2016, 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosgenin: A concise report. Nat. Prod. Bioprospecting 2012, 2, 46–52. [Google Scholar] [CrossRef]
- Zheng, H.; Youdim, M.B.; Weiner, L.M.; Fridkin, M. Novel potential neuroprotective agents with both iron chelating and amino acid-based derivatives targeting central nervous system neurons. Biochem. Pharmacol. 2005, 70, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.; Guo, S.; Yang, L. Synthesis and antitumour activity of arctigenin amino acid ester derivatives against H22 hepatocellular carcinoma. Nat. Prod. Res. 2018, 32, 406–411. [Google Scholar] [CrossRef]
- Wu G, R.; Xu, B.; Yang Y, Q. Synthesis and biological evaluation of podophyllotoxin derivatives as selective antitumor agents. Eur. J. Med. Chem. 2018, 155, 183–196. [Google Scholar] [CrossRef]
- Gong, G.; Qin, Y.; Huang, W. Anti-thrombosis effect of diosgenin extract from Dioscorea zingiberensis CH Wright in vitro and in vivo. Phytomedicine 2011, 18, 458–463. [Google Scholar] [CrossRef]
- Lee, B.K.; Kim, C.J.; Shin, M.S.; Cho, Y.S. Diosgenin improves functional recovery from sciatic crushed nerve injury in rats. J. Exerc. Rehabil. 2018, 14, 566. [Google Scholar] [CrossRef]
- Dias, K.L.G.; de Azevedo Correia, N.; Pereira, K.K.G.; Barbosa-Filho, J.M.; Cavalcante, K.V.M.; Araújo, I.G.A.; Medeiros, I.A. Mechanisms involved in the vasodilator effect induced by diosgenin in rat superior mesenteric artery. Eur. J. Pharmacol. 2007, 574, 172–178. [Google Scholar] [CrossRef]
- Zheng, H.; Wei, Z.; Xin, G.; Ji, C.; Wen, L.; Xia, Q.; Huang, W. Preventive effect of a novel diosgenin derivative on arterial and venous thrombosis in vivo. Bioorganic Med. Chem. Lett. 2016, 26, 3364–3369. [Google Scholar] [CrossRef]
- Wei, Z.; Xin, G.; Wang, H.; Zheng, H.; Ji, C.; Gu, J.; Huang, W. The diosgenin prodrug nanoparticles with pH-responsive as a drug delivery system uniquely prevents thrombosis without increased bleeding risk. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 673–684. [Google Scholar] [CrossRef]
- Okawara, M.; Hashimoto, F.; Todo, H.; Sugibayashi, K.; Tokudome, Y. Effect of liquid crystals with cyclodextrin on the bioavailability of a poorly water-soluble compound, diosgenin, after its oral administration to rats. Int. J. Pharm. 2014, 472, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, F.; Yang, H.; Li, R.; Guo, H.; Hu, L. Diosgenin glucoside provides neuroprotection by regulating microglial M1 polarization. Int. Immunopharmacol. 2017, 50, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, B.N.; Le, H.T.; Hong, H.N.; Lim, C.W.; Park, K.H.; Kang, T.H. Small molecular weight PEGylation of diosgenin in an in vivo animal study for diabetic auditory impairment treatment. Bioorganic Med. Chem. Lett. 2012, 22, 4609–4612. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, R.; Gottis, S.; Meyer, C.; Grand, E.; Deveaux, V.; Kovensky, J.; Stasik, I. Cholesteryl and diosgenyl glycosteroids: Synthesis and characterization of new smectic liquid crystals. Carbohydr. Res. 2015, 404, 70–78. [Google Scholar] [CrossRef]
- Deng, S.; Yu, B.; Hui, Y.; Yu, H.; Han, X. Synthesis of three diosgenyl saponins: Dioscin, polyphyllin D and balanitin 7. Carbohydr. Res. 1999, 317, 53–62. [Google Scholar] [CrossRef]
- Ullah, A.; Iftikhar, F.; Arfan, M.; Batool Kazmi, S.T.B.; Anjum, M.N.; Haq, I.U.; Haq, I.; Ayaz, M.; Farooq, S.; Rashid, U. Amino acid conjugated antimicrobial drugs: Synthesis, lipophilicity- activity relationship, antibacterial and urease inhibition activity. Eur. J. Med. Chem. 2018, 145, 140–153. [Google Scholar] [CrossRef]
- Mi, K.K.; Park, K.S.; Yeo, W.S.; Choo, H.; Chong, Y. In vitro solubility, stability and permeability of novel quercetin-amino acid conjugates. Bioorganic Med. Chem. 2009, 17, 1164–1171. [Google Scholar]
- Suhas, R.; Chandrashekar, S.; Gowda, D.C. Synthesis of uriedo and thiouriedo derivatives of peptide conjugated heterocycles -A new class of promising antimicrobials. Eur. J. Med. Chem. 2012, 48, 179–191. [Google Scholar] [CrossRef]
- Vardhan, D.M.S.; Shantharam, C.S.; Ramesh, S.; Sridhara, M.B.; Gowda, D.C. Synthesis and SAR studies of urea and thiourea derivatives of gly/pro conjugated to piperazine analogue as potential AGE inhibitors. Protein Pept. Lett. 2013, 20, 888–897. [Google Scholar] [CrossRef]
- Fang, L.; Wang, M.; Gou, S.; Liu, X.; Zhang, H.; Cao, F. Combination of amino acid/dipeptide with nitric oxide donating oleanolic acid derivatives as PepT1 targeting antitumor prodrugs. J. Med. Chem. 2014, 57, 1116–1120. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C.; Valderrama, J.A. Gastroprotective effect and cytotoxicity of labdeneamides with amino acids. Planta Med. 2011, 77, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Zhang, X.H.; Han, Y.T.; Wu, G.R.; Cai, D.S.; Xue, N.N.; Guo, W.B.; Yang, Y.Q.; Chen, M.; Zhang, X.Y.; et al. Design, Synthesis and Cytotoxic Analysis of Novel Hederagenin–Pyrazine Derivatives Based on Partial Least Squares Discriminant Analysis. Int. J. Mol. Sci. 2018, 19, 2994. [Google Scholar]
- Tanabe, M.; Nitta, A.; Ono, H. Neuroprotection via strychnine-sensitive glycine receptors during post-ischemic recovery of excitatory synaptic transmission in the hippocampus. J. Pharmacol. Sci. 2010, 1007220421. [Google Scholar] [CrossRef]
- Pinto, M.C.X.; Simão, F.; da Costa, F.L.P.; Rosa, D.V.; de Paiva, M.J.N.; Resende, R.R.; Romano-Silva, M.A.; Gomez, M.V.; Gomez, R.S. Sarcosine preconditioning induces ischemic tolerance against global cerebral ischemia. Neuroscience 2014, 271, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.X.; Mourão, F.A.G.; Binda, N.S.; Leite, H.R.; Gomez, M.V.; Massensini, A.R.; Gomez, R.S. Pharmacological induction of ischemic tolerance in hippocampal slices by sarcosine preconditioning. Neurochem. Int. 2012, 61, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.H.; Kim, S.H. Role of Oxidative Stress in Neurodegenerative Diseases and Ischemic Stroke and Prospects of Antioxidant Therapies as a New Therapeutic Strategy. Hanyang Med. Rev. 2006, 26, 133–143. [Google Scholar]
- Sun, G.; Wang, J.; Guo, X.; Lei, M.; Zhang, Y.; Wang, X.; Hu, L. Design, synthesis and biological evaluation of LX2343 derivatives as neuroprotective agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 145, 622–633. [Google Scholar] [CrossRef]
- Seetapun, S.; Yaoling, J.; Wang, Y.; Zhu, Y.Z. Neuroprotective effect of Danshensu derivatives as anti-ischaemia agents on SH-SY5Y cells and rat brain. Biosci. Rep. 2013, 33, 677–688. [Google Scholar] [CrossRef]
- Shin, E.S.; Kim, Y.J.; Kang, K.A.; Lee, J.; Jeon, J.Y. Effects of Erythropoietin in Hypoxia-Induced Ischemia on Differentiated Human Neuroblastoma SH-SY5Y and Rat Stroke Model. Korean J. Psychopharmacol. 2010, 21, 22–28. [Google Scholar]
- Liu, W.; Feng, G.S.; Ou, Y.; Xu, J.; Zhang, Z.J.; Zhang, G.X.; Jiang, J. Neuroprotective effect of apocynin nitrone in oxygen glucose deprivation-treated SH-SY5Y cells and rats with ischemic stroke. Trop. J. Pharm. Res. 2016, 15, 1681–1689. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Chen, Y.; Liang, H.; Cheng, C.; He, Q. Neuroprotective effects of chloroform and aqueous fractions of noni juice against t-Butyl hydroperoxide-induced oxidative damage in SH-SY5Y cells. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Abd-Alla, H.I.; Mahmoud, K.H.; El-Toumy, S.A. In vitro anti-nitrosative, antioxidant and cytotoxicity activities of plant flavonoids: A comparative study. Med. Chem. Res. 2014, 23, 3298–3307. [Google Scholar] [CrossRef]
- Richardson, M.; Singh, G. Observations on the use of the avian chorioallantoic membrane (CAM) model in investigations into angiogenesis. Curr. Drug Targets-Cardiovasc. Hematol. Disord. 2003, 3, 155–185. [Google Scholar] [CrossRef]
- Kue, C.S.; Tan, K.Y.; LaM, M.L.; Lee, H.B. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp. Anim. 2015, 14-0059. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Nampoothiri, S.S.; Rajanikant, G.K. Folic acid exerts post-ischemic neuroprotection in vitro through HIF-1α stabilization. Mol. Neurobiol. 2018, 55, 8328–8345. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, Z.; Wei, X.; Chen, Z.; Fu, Y.; Yang, X.; Li, M. Cystatin C as a potential therapeutic mediator against Parkinson’s disease via VEGF-induced angiogenesis and enhanced neuronal autophagy in neurovascular units. Cell Death Dis. 2017, 8, e2854. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, S.; Liu, X.; Tang, F.; Zhao, P.; Cheng, K.; Feng, W. Protective effect of troxerutin and cerebroprotein hydrolysate injection on cerebral ischemia through inhibition of oxidative stress and promotion of angiogenesis in rats. Mol. Med. Rep. 2019, 19, 3148–3158. [Google Scholar] [CrossRef]
- Li, B.; Yan, W.; Zhang, C.; Zhang, Y.; Liang, M.; Chu, F.; Gong, Y.; Xu, B.; Wang, P.; Lei, H. New synthesis method for sultone derivatives: Synthesis, crystal structure and biological evaluation of S-CA. Molecules 2015, 20, 4307–4318. [Google Scholar] [CrossRef]
- Wang, P.L.; Cheng, Y.T.; Xu, K.; An, Y.W.; Wang, W.; Li, Q.S.; Zhang, H.G.; Lei, H.M. Synthesis and Antitumor Evaluation of One Novel Tetramethylpyrazine-Rhein Derivative. Asian J. Chem. 2013, 25, 4885–4888. [Google Scholar] [CrossRef]
- Wang, P.; She, G.; Yang, Y.; Li, Q.; Zhang, H.; Liu, J.; Gao, Y.; Xu, X.; Lei, H. Synthesis and biological evaluation of new ligustrazine derivatives as anti-tumor agents. Molecules 2012, 17, 4972–4985. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.G.; Li, Y.; Wang, L.; Xu, Y.X.; Gautam, S.C.; Chopp, M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 2003, 92, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.F.; Yin, H.; Yao, Y.Y.; Borlongan, C.V.; Chao, L.; Chao, J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum. Gene Ther. 2006, 17, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sobrino, M.; Rosell, A.; Hernández-Guillamon, M.; Penalba, A.; Boada, C.; Domingues-Montanari, S.; Montaner, J. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis 2011, 216, 205–211. [Google Scholar] [CrossRef]
- Beck, H.; Plate, K.H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009, 117, 481–496. [Google Scholar] [CrossRef]
- Grasso, G.; Santoro, A.M.; Lanza, V.; Sbardella, D.; Tundo, G.R.; Ciaccio, C.; Marini, S.; Coletta, M.; Milardi, D. The double faced role of copper in Aβ homeostasis: A survey on the interrelationship between metal dyshomeostasis, UPS functioning and autophagy in neurodegeneration. Coord. Chem. Rev. 2017, 347, 1–22. [Google Scholar] [CrossRef]
- Arena, G.; Fattorusso, R.; Grasso, G.; Grasso, G.I.; Isernia, C.; Malgieri, G.; Milardi, D.; Rizzarelli, E. Zinc (II) complexes of ubiquitin: Speciation, affinity and binding features. Chem. A Eur. J. 2011, 17, 11596–11603. [Google Scholar] [CrossRef]
- Milewski, K.; Hilgier, W.; Fręśko, I.; Polowy, R.; Podsiadłowska, A.; Zołocińska, E.; Grymanowska, A.W.; Filipkowski, R.K.; Albrecht, J.; Zielińska, M. Carnosine reduces oxidative stress and reverses attenuation of righting and postural reflexes in rats with thioacetamide-induced liver failure. Neurochem. Res. 2016, 41, 376–384. [Google Scholar] [CrossRef]
- Drąg-Zalesińska, M.; Drąg, M.; Poręba, M.; Borska, S.; Kulbacka, J.; Saczko, J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45). Cancer Cell Int. 2017, 17, 4. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 1–18 are available from the authors. |






| Compound | R | Structure |
|---|---|---|
| DG-1 | BOC-Gly |  |
| DG-2 | BOC-L-Ala |  |
| DG-3 | BOC- Sar |  |
| DG-4 | BOC-L-Pro |  |
| DG-5 | BOC-L-Leu |  |
| DG-6 | BOC-L-Ile |  |
| DG-7 | BOC-L-Phe |  |
| DG-8 | CBZ-L-Val |  |
| DG-9 | CBZ-L-Lys |  |
| DG-10 | Gly |  |
| DG-11 | L-Ala |  |
| DG-12 | Sar |  |
| DG-13 | L-Pro |  |
| DG-14 | L-Leu |  |
| DG-15 | L-Ile |  |
| DG-16 | L-Phe |  |
| DG-17 | L-Val |  |
| DG-18 | L-Lys |  |
| Compound | Viability Rate (%) | EC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 3.12 μM | 6.25 μM | 12.50 μM | 25.00 μM | 50.00 μM | ||
| Edaravone | 4.72 | 49.77 | 50.76 | 40.14 | 37.58 | 21.60 ± 3.04 |
| DG | −4.01 | 26.90 | 7.75 | 16.13 | −4.80 | >50 |
| DG-1 | −11.91 | 3.91 | 32.24 | 9.03 | 22.63 | >50 |
| DG-2 | 17.31 | 4.90 | 10.41 | 17.66 | 19.36 | >48.70 ± 1.91 |
| DG-3 | 0.64 | 29.63 | 52.76 | 94.40 | 45.78 | 15.20 ± 0.96 |
| DG-4 | −7.16 | 35.57 | 69.78 | 57.48 | 22.39 | 23.84 ± 6.83 |
| DG-5 | 17.11 | 69.32 | 74.71 | 82.06 | 47.86 | 9.51 ± 1.79 |
| DG-6 | 22.14 | 27.13 | 51.78 | 50.45 | 49.06 | 18.51 ± 1.87 |
| DG-7 | −13.26 | 2.93 | 2.14 | 5.74 | −11.19 | >50 |
| DG-8 | −7.49 | 12.93 | 49.74 | 35.24 | 14.87 | 38.09 ± 6.68 |
| DG-9 | −6.02 | 25.03 | 49.87 | 36.28 | 8.33 | 36.24 ± 8.67 |
| DG-10 | −5.22 | 17.87 | 12.57 | 5.55 | 0.08 | >50 |
| DG-11 | −11.63 | 10.33 | 15.08 | 12.42 | 20.15 | >50 |
| DG-12 | −25.18 | 42.36 | 38.07 | 24.61 | −14.68 | >50 |
| DG-13 | 13.96 | 38.79 | 68.85 | 93.98 | 89.62 | 7.22 ± 1.58 |
| DG-14 | −5.77 | 43.72 | 20.36 | 16.98 | 49.88 | 32.58 ± 0.97 |
| DG-15 | 24.16 | 68.32 | 88.96 | 76.26 | 75.56 | 6.86 ± 0.69 |
| DG-16 | 16.35 | 51.68 | 74.31 | 65.76 | 60.61 | 11.16 ± 2.62 |
| DG-17 | −3.99 | −3.65 | 1.00 | −0.76 | −6.77 | >50 |
| DG-18 | −37.10 | −53.53 | −97.67 | −103.66 | −107.92 | >50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.; Qi, J.; Yang, Y.; Zhang, W.; Zhou, F.; Jia, X.; Guo, W.; Huang, X.; Gao, F.; Chen, H.; et al. Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis. Molecules 2019, 24, 4025. https://doi.org/10.3390/molecules24224025
Cai D, Qi J, Yang Y, Zhang W, Zhou F, Jia X, Guo W, Huang X, Gao F, Chen H, et al. Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis. Molecules. 2019; 24(22):4025. https://doi.org/10.3390/molecules24224025
Chicago/Turabian StyleCai, Desheng, Jinchai Qi, Yuqin Yang, Wenxi Zhang, Fei Zhou, Xiaohui Jia, Wenbo Guo, Xuemei Huang, Feng Gao, Hongshan Chen, and et al. 2019. "Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis" Molecules 24, no. 22: 4025. https://doi.org/10.3390/molecules24224025
APA StyleCai, D., Qi, J., Yang, Y., Zhang, W., Zhou, F., Jia, X., Guo, W., Huang, X., Gao, F., Chen, H., Li, T., Li, G., Wang, P., Zhang, Y., & Lei, H. (2019). Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis. Molecules, 24(22), 4025. https://doi.org/10.3390/molecules24224025