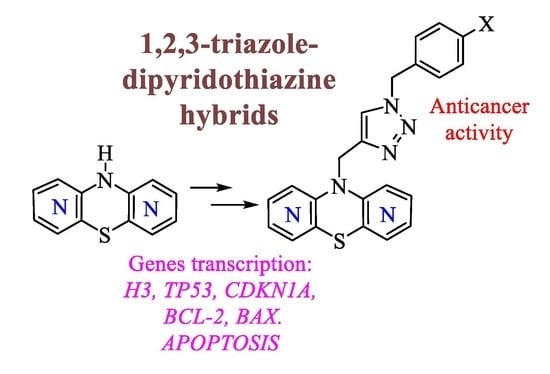
Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Anticancer Activity
2.3. Apoptosis Assay
3. Materials and Methods
3.1. Chemistry
3.2. Anticancer Effects In Vitro
3.2.1. Cell Culture
3.2.2. Cell Proliferation and Viability
3.2.3. The RT-qPCR Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, S.C. Phenothiazine: The Parent molecule. Curr. Drug Targ. 2006, 7, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Viveiros, M. Thioridazine: A Non-Antibiotic Drug Highly Effective, in Combination with First Line Anti-Tuberculosis Drugs, against Any Form of Antibiotic Resistance of Mycobacterium tuberculosis Due to Its Multi-Mechanisms of Action. Antibiotics 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Huang, D.; Qin, L.; Zheng, Z.; Hua, L.; Wang, G.; Huang, J.; Huang, H. Targeting Lung Cancer Stem Cells with Antipsychological Drug Thioridazine. BioMed Res. Int. 2016, 2016, 6709828. [Google Scholar] [CrossRef] [PubMed]
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am. J. Ther. 2006, 13, 261–273. Available online: https://journals.lww.com/americantherapeutics/toc/2006/05000 (accessed on 12 June 2019). [CrossRef]
- Dasgupta, A.; Dastridara, S.G.; Shirataki, Y.; Motohashi, N. Antibacterial activity of artificial phenothiazines and isoflavones from plants. Top Heterocycl. Chem. 2008, 15, 67–132. [Google Scholar]
- Aaron, J.J.; Gaye Seye, M.D.; Trajkovska, S.; Motohashi, N. Bioactive Phenothiazines and Benzo[a]phenothiazines: Spectroscopic studies and biological and biomedical properties and applications. Top Heterocycl. Chem. 2009, 16, 153–231. [Google Scholar]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. J. Heterocycl. Chem. 2009, 46, 355–391. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M.; Zaczyńska, E. Azaphenothiazines a promising phenothiazine derivatives. An insight into nomenclature, synthesis, structure elucidation and biological properties. Eur. J. Med. Chem. 2017, 138, 774–806. [Google Scholar] [CrossRef]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 2011, 46, 3179–3189. [Google Scholar] [CrossRef]
- Jaszczyszyn, A.; Gąsiorowski, K.; Świątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. Chemical structure of phenothiazines and their biological activity. Pharmacol. Rep. 2012, 64, 16–23. Available online: http://www.if-pan.krakow.pl/pjp/pdf/2012/1_16.pdf (accessed on 12 June 2019). [CrossRef]
- Jeleń, M.; Pluta, K.; Latocha, M.; Morak-Młodawska, B.; Suwińska, K.; Kusmierz, D. Evaluation of angularly condensed diquinothiazines as potential anticancer agents. Bioorg. Chem. 2019, 87, 810–820. [Google Scholar] [CrossRef]
- González-Muñoz, G.C.; Arce, M.P.; López, B.; Pérez, C.; Villarroya, M.; López, M.G.; García, A.G.; Conde, S.; Rodríguez-Franco, M.I. Old phenothiazine and dibenzothiadiazepine derivatives for tomorrow’s neuroprotective therapies against neurodegenerative diseases. Eur. J. Med. Chem. 2010, 45, 6152–6158. [Google Scholar] [CrossRef] [PubMed]
- Strządala, L.; Fiedorowicz, A.; Wysokińska, E.; Zioło, E.; Grudzień, M.; Jeleń, M.; Pluta, K.; Morak-Młodawska, B.; Zimecki, M.; Kałas, W. An Anti-Inflammatory Azaphenothiazine Inhibits Interferon β Expression and CXCL10 Production in KERTr Cells. Molecules 2018, 23, 2433. [Google Scholar] [CrossRef] [PubMed]
- Presti, M.; Bazán, P.; Strauss, M.; Báez, A.; Rivarola, H.; Paglini-Oliva, P. Trypanothione reductase inhibitors: Overview of the action of thioridazine in different stages of Chagas disease. Acta Trop. 2015, 145, 79–87. [Google Scholar] [CrossRef]
- Spengler, G.; Csonka, A.; Molnar, J.; Amaral, L. The anticancer activity of the old neuroleptic phenothiazine-type drug thioridazine. Anticancer Res. 2016, 36, 5701–5706. [Google Scholar] [CrossRef]
- Liu, N.; Jin, Z.; Zhang, J.; Jin, J. Antitumor evaluation of novel phenothiazine derivatives that inhibit migration and tubulin polymerization against gastric cancer MGC-803 cells. Investig. New Drugs 2018, 37, 188–198. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M. Anticancer activity of newly synthesized azaphenothiazines in NCI’s anticancer screening. Pharmacol. Rep. 2010, 62, 319–332. Available online: http://www.if-pan.krakow.pl/pjp/pdf/2010/2_319.pdf (accessed on 12 June 2019). [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Matralis, A.N.; Kourounakis, A.P. Antioxidant Activity of Newly Synthesized 2, 7-Diazaphenothiazines. Arch. Pharm. Int. J. Pharm. Med. Chem. 2010, 343, 268–273. [Google Scholar] [CrossRef]
- Zimecki, M.; Artym, J.; Kocięba, M.; Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Immunosupressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell. Mol. Biol. Lett. 2009, 14, 622–635. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1, 8-diazaphenothiazines. Med. Chem. Res. 2015, 24, 1408–1418. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis and anticancer and lipophilic properties of 10-dialkylaminobutynyl derivatives of 1, 8- and 2, 7-diazaphenothiazines. J. Enzyme Inhib. Med. Chem. 2016, 31, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M. Synthesis, spectroscopic characterization, and anticancer activity of new 10-substituted 1, 6-diazaphenothiazines. Med. Chem. Res. 2016, 25, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Suwińska, K.; Jeleń, M.; Kuśmierz, D. 3, 6-Diazaphenothiazines as potential lead molecules-synthesis, characterization and anticancer activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Wenzhi, Z.; Okechukwu, P.N.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Md Akim, A.; Ang, K.-P.; Ooi, K.K. 10H-3, 6-Diazaphenothiazines Induce G2/M Phase Cell Cycle Arrest, Caspase-dependent Apoptosis and Inhibits Cell Invasion of A2780 Ovarian Carcinoma Cells through Regulation on NF-κB and [BIRC6-XIAP] Complexes. Drug Des. Dev. Ther. 2017, 11, 3045–3063. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis, anticancer activity and apoptosis induction of novel 3, 6-diazaphenothiazines. Molecules 2019, 24, 267. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1, 2, 3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Duan, Y.C.; Ma, Y.C.; Zhang, E.; Shi, X.J.; Wang, M.M.; Ye, X.W.; Liu, H.M. Design and synthesis of novel 1, 2, 3-triazole-dithiocarbamate hybrids as potential anticancer agents. Eur. J. Med. Chem. 2013, 62, 11–19. [Google Scholar] [CrossRef]
- Totobennazara, J.; Burke, J.A. New click-chemistry methods for 1, 2, 3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Upadhyay, S.; Singh, J.; Sahu, V. A novel approach to the synthesis of 1, 2, 3-triazoles and their SAR studies. Med. Chem. Res. 2010, 19, 589–602. [Google Scholar] [CrossRef]
- Vatmurge, N.S.; Hazra, B.G.; Pore, V.S.; Shirazi, F.; Chavan, P.S.; Deshpande, M.V. Synthesis and antimicrobial activity of lactam-bile acid conjugates linked via triazole. Bioorg. Med. Chem. Lett. 2008, 18, 2043–2047. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jóźwiak, K. Click Chemistry for Drug Development and Diverse Chemical-Biology Application. Chem. Rev. 2013, 113, 4905–49792. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Ahmad, S.; Alam, M.S. Bioactive triazoles: A potential review. J. Chem. Pharm. Res. 2012, 4, 5157–5164. [Google Scholar]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: Apromising antitubercular agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Keskar, K. A Robust, Well-Defined Homogeneous Silver(I) Catalyst for MildIntramolecular Hydroamination of 2-Ethynylanilines Leading to Indoles. Eur. J. Org. Chem. 2014, 2014, 1622–1629. [Google Scholar] [CrossRef]
- Allen, M.A.; Andrysik, Z.; Dengler, V.L.; Mellert, H.S.; Guarnieri, A.; Freeman, J.A.; Sullivan, K.D.; Galbraith, M.D.; Luo, X.; Dowell, R.D.; et al. Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. Elife 2014, 3, 1–29. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Beberok, A.; Wrześniok, D.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Ciprofloxacin triggers the apoptosis of human triple-negative breast cancer MDA-MB-231 cells via the p53/Bax/Bcl-2 signaling pathway. Int. J. Oncol. 2018, 52, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- Beberok, A.; Rzepka, Z.; Respondek, M.; Rok, J.; Sierotowicz, D.; Wrześniok, D. GSH depletion, mitochondrial membrane breakdown, caspase-3/7 activation and DNA fragmentation in U87MG glioblastoma cells: New insight into the mechanism of cytotoxicity induced by fluoroquinolones. Eur. J. Pharmacol. 2018, 835, 94–107. [Google Scholar] [CrossRef]
- Dutto, I.; Tillhon, M.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Biology of the cell cycle inhibitor p21 CDKN1A: Molecular, mechanisms and relevance in chemical toxicology. Arch. Toxicol. 2015, 89, 155–178. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Hemann, M.T.; Lowe, S.W. The p53-Bcl-2 connection. Cell Death Differ. 2006, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds. 1b–4f. are available from the authors. |


| 1H NMR | ROESY | COSY |
|---|---|---|
| 5.22 CH2NTh | 5.22/7.46 | 5.22/7.61 |
| 5.49 CH2NTr | 5.49/7.23/7.61 | 5.49/7.23/7.61 |
| 6.76 H3 | 6.76/7.23/7.92 | 6.76/7.23/7.92 |
| 6.93 H8 | 6.93/7.46 | 6.93/7.46/7.92 |
| 7.23 o-HPh, 1H4 | 7.23/6.76/7.35 | 7.23/6.76/7.35/7.92 |
| 7.35 m,p-HPh | 7.35/7.23 | 7.35/7.23 |
| 7.46 H9 | 7.61/5.22 | 7.46/6.93/7.92 |
| 7.61 HTr | 7.61/5.49/7.23 | 7.61/5.22 |
| 7.92 H7 + H2 | 7.92/6.76/6.93 | 7.92/6.76/6.93/7.23/7.46 |
| No | Anticancer Activity IC50 (μM) | ||||
|---|---|---|---|---|---|
| SNB-19 | Caco-2 | A549 | MDA-MB231 | NHDF | |
| 1b | 69.15+/−3.14 | 19.92+/-2.71 | >100 | 13.66+/−1.16 | >100 |
| 1c | 5.72+/−1.44 | 8.10+/−0.92 | 29.12+/−2.59 | 7.82+/−1.32 | >100 |
| 1d | 4.66+/−1.16 | 0.25+/−0.02 | 0.25+/−0.01 | 0.51+/−0.09 | >100 |
| 1e | 9.65+/−1.99 | 79.65+/−4.12 | 1.79+/−0.62 | 25.79+/−1.32 | >100 |
| 1f | >100 | >100 | >100 | 68.68+/−5.12 | >100 |
| 2b | 41.91+/−3.15 | >100 | 77.44+/−3.22 | 56.34+/−5.72 | >100 |
| 2c | 55.23+/−5.78 | 43.61+/−6.52 | 60.23+/−2.77 | 9.21+/−3.01 | >100 |
| 2d | 59.87+/−9.99 | 11.42+/−2.13 | 1.82+/−0.71 | 4.71+/−0.22 | 42.95+/−5.11 |
| 2e | 10.62+/−1.11 | 34.38+/−2.72 | 63.75+/−2.78 | 12.04+/−2.21 | >100 |
| 2f | 56.34+/−5.72 | 32.55+/−8.21 | 46.80+/−2.73 | 12.04+/−6.11 | >100 |
| 3b | 2.04+/−0.21 | 0.26+/−0.01 | 0.26+/−0.11 | 0.77+/−0.10 | 22.66+/−1.32 |
| 3c | 57.92+/−4.96 | 29.87+/−5.21 | 27.23+/−2.16 | 25.66+/−4.11 | 70.61+/−12.21 |
| 3d | 31.31+/−10.99 | 33.07+/−9.18 | 17.91+/−1.11 | 4.26+/−0.15 | >100 |
| 3e | 26.80+/−1.72 | 10.02+/−0.99 | 30.07+/−3.21 | 3.50+/−0.35 | 49.47+/−1.11 |
| 3f | 32.58+/−2.77 | 14.54+/−1.22 | 0.65+/−0.10 | 0.64+/−0.10 | 52.90+/−3.21 |
| 4b | 20.80+/−1.96 | 57.90+/−2.15 | 60.62+/−3.52 | 7.87+/−1.11 | >100 |
| 4c | 6.25+/−0.62 | 0.25+/−0.02 | 0.25+/−0.01 | 1.97+/−0.13 | 2.85+/−0.34 |
| 4d | 14.87+/−1.70 | 42.06+/−2.99 | 53.99+/−10.72 | 13.44+/−1.22 | >100 |
| 4e | 53.62+/−3.72 | 74.28+/−4.72 | 42.69+/−13.72 | 38.77+/−2.12 | >100 |
| 4f | 44.83+/−13.62 | 67.52+/−11.87 | >100 | 44.33+/−11.79 | >100 |
| Cisplatin | 3.73+/−0.62 | 10.53+/−1.52 | 0.60+/−0.11 | 3.13+/−0.24 | 63.87+/−1.32 |
| gene | SNB-19 | Caco-2 | A549 | MDA-MB231 | |
|---|---|---|---|---|---|
| Number of mRNA Copies/μg Total RNA | |||||
| H3 | control | 143+/−41 | 11+/−3 | 23+/-13 | 2+/−1 |
| 1d | 52+/−11 | 45+/−3 | 18+/−5 | 8+/−6 | |
| TP53 | control | 198+/−52 | 65+/−24 | 49+/−17 | 56+/−16 |
| 1d | 128+/−24 | 193+/−16 | 54+/−19 | 46+/−20 | |
| CDKN1A | control | 698+/−34 | 266+/−49 | 1012+/−143 | 710+/−486 |
| 1d | 308+/−51 | 260+/−21 | 969+/−137 | 577+/−308 | |
| BCL-2 | control | 70604+/−7852 | 673718+/−52998 | 22485+/−4794 | 207802+/−15572 |
| 1d | 17497+/−4607 | 723708+/−217134 | 15631+/−2296 | 16495+/−4607 | |
| BAX | control | 995+/−98 | 1062+/−141 | 1181+/−132 | 835+/−63 |
| 1d | 220+/−18 | 969+/−264 | 835+/−231 | 221+/−16 | |
| BCL-2/BAX | control | 71 | 634 | 19 | 249 |
| 1d | 80 | 743 | 18 | 80 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents. Molecules 2019, 24, 4388. https://doi.org/10.3390/molecules24234388
Morak-Młodawska B, Pluta K, Latocha M, Jeleń M, Kuśmierz D. Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents. Molecules. 2019; 24(23):4388. https://doi.org/10.3390/molecules24234388
Chicago/Turabian StyleMorak-Młodawska, Beata, Krystian Pluta, Małgorzata Latocha, Małgorzata Jeleń, and Dariusz Kuśmierz. 2019. "Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents" Molecules 24, no. 23: 4388. https://doi.org/10.3390/molecules24234388
APA StyleMorak-Młodawska, B., Pluta, K., Latocha, M., Jeleń, M., & Kuśmierz, D. (2019). Design, Synthesis, and Structural Characterization of Novel Diazaphenothiazines with 1,2,3-Triazole Substituents as Promising Antiproliferative Agents. Molecules, 24(23), 4388. https://doi.org/10.3390/molecules24234388