Exploring the Self-Assembled Tacticity in Aurophilic Polymeric Arrangements of Diphosphanegold(I) Fluorothiolates
Abstract
:1. Introduction
2. Results
3. Discussion
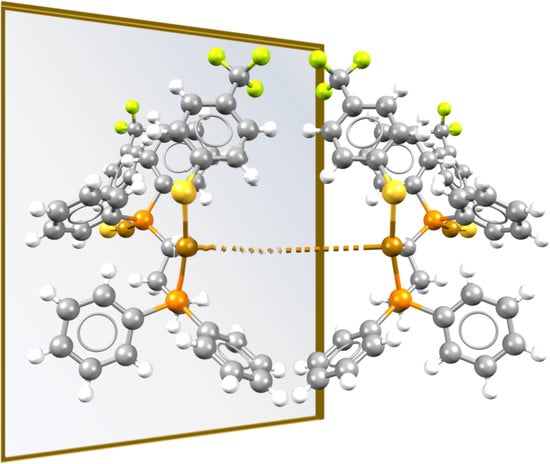
3.1. Isotactic Polymers
3.2. Syndiotactic Polymers
4. Conclusions
5. Materials and Methods
5.1. Synthesis and Characterization
5.2. Crystal Structure Determination
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laguna, A. Modern Supramolecular Gold Chemistry; Laguna, A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 9783527623778. [Google Scholar]
- Tiekink, E.R.T. Supramolecular assembly of molecular gold(I) compounds: An evaluation of the competition and complementarity between aurophilic (Au⋯Au) and conventional hydrogen bonding interactions. Coord. Chem. Rev. 2014, 275, 130–153. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931. [Google Scholar] [CrossRef] [PubMed]
- Bardají, M. Gold Liquid Crystals in the XXI Century. Inorganics 2014, 2, 433–454. [Google Scholar] [CrossRef] [Green Version]
- Yam, V.W.-W.; Cheng, E.C.-C. Highlights on the recent advances in gold chemistry--a photophysical perspective. Chem. Soc. Rev. 2008, 37, 1806–1813. [Google Scholar] [CrossRef]
- Ai, P.; Mauro, M.; De Cola, L.; Danopoulos, A.A.; Braunstein, P. A Bis(Diphosphanyl N-Heterocyclic Carbene) Gold Complex: A Synthon for Luminescent Rigid AuAg2 Arrays and Au5 and Cu6 Double Arrays. Angew. Chemie Int. Ed. 2016, 55, 3338–3341. [Google Scholar] [CrossRef]
- Lima, J.; Rodríguez, L.; Lima, J.C.; Rodríguez, L. Supramolecular Gold Metallogelators: The Key Role of Metallophilic Interactions. Inorganics 2014, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.J.; Sakai, K.; Leznoff, D.B. The use of aurophilic and other metal–metal interactions as crystal engineering design elements to increase structural dimensionality. Chem. Soc. Rev. 2008, 37, 1884. [Google Scholar] [CrossRef]
- Wan, Q.; Xia, J.; Lu, W.; Yang, J.; Che, C.-M. Kinetically Controlled Self-Assembly of Phosphorescent Au III Aggregates and Ligand-to-Metal–Metal Charge Transfer Excited State: A Combined Spectroscopic and DFT/TDDFT Study. J. Am. Chem. Soc. 2019, 141, 11572–11582. [Google Scholar] [CrossRef]
- Shakirova, J.R.; Grachova, E.V.; Karttunen, A.J.; Gurzhiy, V.V.; Tunik, S.P.; Koshevoy, I.O. Metallophilicity-assisted assembly of phosphine-based cage molecules. Dalt. Trans. 2014, 43, 6236. [Google Scholar] [CrossRef] [Green Version]
- Deäk, A.; Tunyoginyi, T.; Tärkä, G.; Kiräly, P.; Pälinkäs, G. Self-assembly of gold(I) with diphosphine and bitopic nitrogen donor linkers in the presence of trifluoroacetate anion: Formation of coordination polymer versus discrete macrocycle. CrystEngComm 2007. [Google Scholar] [CrossRef]
- Blasco, D.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pascual, D.; Rodríguez-Castillo, M. Cooperative Au(I)···Au(I) Interactions and Hydrogen Bonding as Origin of a Luminescent Adeninate Hydrogel Formed by Ultrathin Molecular Nanowires. Inorg. Chem. 2018, 57, 3805–3817. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef] [Green Version]
- Mendizabal, F.; Pyykkö, P. Aurophilic attraction in binuclear complexes with Au(i) and Au(iii). A theoretical study. Phys. Chem. Chem. Phys. 2004, 6, 900–905. [Google Scholar] [CrossRef]
- Brands, M.B.; Nitsch, J.; Guerra, C.F. Relevance of Orbital Interactions and Pauli Repulsion in the Metal–Metal Bond of Coinage Metals. Inorg. Chem. 2018, 57, 2603–2608. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Alcántar, G.; Romo-Islas, G.; Flores-Álamo, M.; Torrens, H. Aurophilicity vs. thiophilicity: Directing the crystalline supramolecular arrangement in luminescent gold compounds. New J. Chem. 2018, 42, 7845–7852. [Google Scholar] [CrossRef]
- Streitberger, M.; Schmied, A.; Hey-Hawkins, E. Selective Formation of Gold(I) Bis-Phospholane Macrocycles, Polymeric Chains, and Nanotubes. Inorg. Chem. 2014, 53, 6794–6804. [Google Scholar] [CrossRef]
- Andris, E.; Andrikopoulos, P.C.; Schulz, J.; Turek, J.; Růžička, A.; Roithová, J.; Rulíšek, L. Aurophilic Interactions in [(L)AuCl]...[(L′)AuCl] Dimers: Calibration by Experiment and Theory. J. Am. Chem. Soc. 2018, 140, 2316–2325. [Google Scholar] [CrossRef] [Green Version]
- Vreshch, V.; Shen, W.; Nohra, B.; Yip, S.K.; Yam, V.W.-W.; Lescop, C.; Réau, R. Aurophilicity versus mercurophilicity: Impact of d 10-d 10 metallophilic interactions on the structure of metal-rich supramolecular assemblies. Chem. - A Eur. J. 2012, 18, 466–477. [Google Scholar] [CrossRef]
- Forfar, L.C.; Zeng, D.; Green, M.; McGrady, J.E.; Russell, C.A. Probing the Structure, Dynamics, and Bonding of Coinage Metal Complexes of White Phosphorus. Chem. - A Eur. J. 2016, 22, 5397–5403. [Google Scholar] [CrossRef] [Green Version]
- Simler, T.; Braunstein, P.; Danopoulos, A.A. Coinage metal complexes with bridging hybrid phosphine–NHC ligands: Synthesis of di- and tetra-nuclear complexes. Dalt. Trans. 2016, 45, 5122–5139. [Google Scholar] [CrossRef] [Green Version]
- Gallego, M.L.; Guijarro, A.; Castillo, O.; Parella, T.; Mas-Balleste, R.; Zamora, F. Nuclearity control in gold dithiocarboxylato compounds. CrystEngComm 2010, 12, 2332. [Google Scholar] [CrossRef]
- Besenius, P.; Portale, G.; Bomans, P.H.H.; Janssen, H.M.; Palmans, A.R.A.; Meijer, E.W. Controlling the growth and shape of chiral supramolecular polymers in water. Proc. Natl. Acad. Sci. 2010, 107, 17888–17893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorca, Y.; Greciano, E.E.; Valera, J.S.; Gómez, R.; Sánchez, L. Hierarchy of Asymmetry in Chiral Supramolecular Polymers: Toward Functional, Helical Supramolecular Structures. Chem. – A Eur. J. 2019, 25, 5848–5864. [Google Scholar] [CrossRef]
- Veselska, O.; Okhrimenko, L.; Guillou, N.; Podbevšek, D.; Ledoux, G.; Dujardin, C.; Monge, M.; Chevrier, D.M.; Yang, R.; Zhang, P.; et al. An intrinsic dual-emitting gold thiolate coordination polymer, [Au(+I)(p-SPhCO2H)]n, for ratiometric temperature sensing. J. Mater. Chem. C 2017, 5, 9843–9848. [Google Scholar] [CrossRef]
- Mohr, F.; Jennings, M.C.; Puddephatt, R.J. Self-Assembly in Gold(I) Chemistry: A Double-Stranded Polymer with Interstrand Aurophilic Interactions. Angew. Chemie Int. Ed. 2004, 43, 969–971. [Google Scholar] [CrossRef]
- Crespo, O.; Gimeno, M.C.; Laguna, A.; Kulcsar, M.; Silvestru, C. Gold Complexes with the Selenolate Ligand [2-(Me2NCH2)C6H4Se]−. Inorg. Chem. 2009, 48, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Fellows, C.M.; Hellwich, K.-H.; Meille, S.V.; Moad, G.; Nakano, T.; Vert, M. Definitions and notations relating to tactic polymers (IUPAC Provisional Recommendation). Pure Appl. Chem. 2019. [Google Scholar]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P. (Tetrahydrothiophene)Gold(I) or Gold(III) Complexes. Inorganic Syntheses 1989, 85–91. [Google Scholar]
- Ahrland, S.; Dreisch, K.; Norén, B.; Oskarsson, Å. Metal-metal interactions in chain compounds of gold(I): Syntheses and crystal structures of chlorotetrahydrothiophenegold(I), bromotetrahydrothiophenegold(I) and iodotetrahydroselenophenegold(I). Mater. Chem. Phys. 1993, 35, 281–289. [Google Scholar] [CrossRef]
- McAuliffe, C.A.; (Dick) Parish, R.V.; Randall, P.D. Gold(I) complexes of unidentate and bidentate phosphorus-, arsenic-, antimony- and sulphur-donor ligands. J. Chem. Soc. Dalt. Trans. 1979, 1730. [Google Scholar] [CrossRef]
- Peach, M.E. Some reactions of pentafluorothiophenol. Preparation of some pentafluoro-phenylthio metal derivatives. Can. J. Chem. 1968, 46, 2699–2706. [Google Scholar] [CrossRef]
- Shaw, R.A.; Woods, M. Preparation and some properties of lead thiolates. J. Chem. Soc. A Inorganic Phys. Theor. 1971, 1569. [Google Scholar] [CrossRef]
- Fleischer, H.; Heller, C.; Schollmeyer, D. Poly[bis(μ-pentafluorobenzenethiolato)lead(II)]. Acta Crystallogr. Sect. E Struct. Reports Online 2006, 62, m1365–m1367. [Google Scholar] [CrossRef] [Green Version]
- Rae, A.D.; Craig, D.C.; Dance, I.G.; Scudder, M.L.; Dean, P.A.W.; Kmetic, M.A.; Payne, N.C.; Vittal, J.J. The Pseudo-Symmetric Structure of Pb(SPh)2. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 457–465. [Google Scholar] [CrossRef]
- Eichhöfer, A. Four New Lead(II) Thiolate Cluster Complexes - Unexpected Products of a Conventional Synthesis. Eur. J. Inorg. Chem. 2005, 2005, 1683–1688. [Google Scholar] [CrossRef]
- Agilent CrysAlis PRO 2014. Available online: https://www.rigaku.com/en/products/smc/crysalis (accessed on 1 December 2019).
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Compound | dAu–Au (Å) | dAu–P (Å) | dAu–S (Å) | θP–Au–S (°) | |||
|---|---|---|---|---|---|---|---|
| 1 | 3.0924 (7) | 2.254 (2) | 2.263 (1) | 2.308 (2) | 2.309 (1) | 173.59 (7) | 175.72 (7) |
| 2 | 3.0288 (6) | 2.257 (1) | 2.259 (1) | 2.308 (1) | 2.313 (1) | 178.40 (4) | 173.63 (4) |
| 4 | 3.2276 (3) | 2.258 (1) | 2.260 (1) | 2.299 (1) | 2.303 (1) | 175.75 (4) | 169.04 (4) |
| 5 | 3.2071 (5) | 2.259 (1) | 2.272 (2) | 2.302 (1) | 2.301 (2) | 169.93 (5) | 165.83 (5) |
| 9 | 3.1475 (5) | 2.262 (3) | 2.272 (3) | 2.309 (3) | 2.315 (3) | 167.0 (1) | 171.5 (1) |
| 10 | 3.1325 (3) | 2.259 (1) | 2.271 (1) | 2.305 (1) | 2.316 (1) | 169.88 (4) | 172.02 (4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Alcántar, G.; Salazar, L.; Romo-Islas, G.; Flores-Álamo, M.; Torrens, H. Exploring the Self-Assembled Tacticity in Aurophilic Polymeric Arrangements of Diphosphanegold(I) Fluorothiolates. Molecules 2019, 24, 4422. https://doi.org/10.3390/molecules24234422
Moreno-Alcántar G, Salazar L, Romo-Islas G, Flores-Álamo M, Torrens H. Exploring the Self-Assembled Tacticity in Aurophilic Polymeric Arrangements of Diphosphanegold(I) Fluorothiolates. Molecules. 2019; 24(23):4422. https://doi.org/10.3390/molecules24234422
Chicago/Turabian StyleMoreno-Alcántar, Guillermo, Laura Salazar, Guillermo Romo-Islas, Marcos Flores-Álamo, and Hugo Torrens. 2019. "Exploring the Self-Assembled Tacticity in Aurophilic Polymeric Arrangements of Diphosphanegold(I) Fluorothiolates" Molecules 24, no. 23: 4422. https://doi.org/10.3390/molecules24234422
APA StyleMoreno-Alcántar, G., Salazar, L., Romo-Islas, G., Flores-Álamo, M., & Torrens, H. (2019). Exploring the Self-Assembled Tacticity in Aurophilic Polymeric Arrangements of Diphosphanegold(I) Fluorothiolates. Molecules, 24(23), 4422. https://doi.org/10.3390/molecules24234422