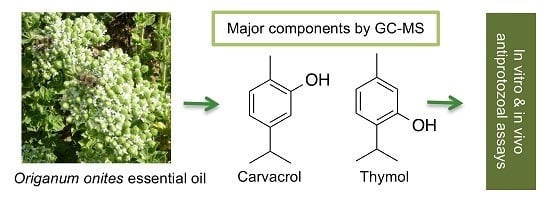
Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the Essential Oil
2.2. In Vitro Antiprotozoal Activity and Cytotoxicity of the Essential Oil
2.3. In Vitro Activity and Cytotoxicity of the Individual Constituents of the Essential Oil
2.4. In Vivo Trypanocidal Activity of Carvacrol and Thymol
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Plant Material and Isolation of the Essential Oil
4.3. Gas Chromatography Analyses
4.4. In Vitro Assay for Plasmodium falciparum
4.5. In Vitro Assay for Trypanosoma brucei rhodesiense
4.6. In Vitro Assay for Trypanosoma cruzi
4.7. In Vitro Assay for Leishmania donovani
4.8. In Vitro Assay for Cytotoxicity on Mammalian Cells
4.9. In Vivo Trypanocidal Activity Assessment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Celep, F.; Dirmenci, T. Systematic and bio-geographic overview of Lamiaceae in Turkey. Nat. Volatiles Essent. Oils (NVEO) 2017, 4, 14–27. [Google Scholar]
- Meyers, M. Oregano and Marjoram: An Herb Society of America Guide to the Genus Origanum; The Herb Society of America: Kirtland, OH, USA, 2005; pp. 105–108. [Google Scholar]
- Boydag, I.; Kurkcuoglu, M.; Ozek, T.; Baser, K.H.C. The isolation of some soluble and dispersed materials of oregano water. Chem. Nat. Compd. 2003, 39, 465–469. [Google Scholar] [CrossRef]
- Baser, K.H.C. The Turkish Origanum species. In Oregano, The Genera Origanum and Lippia; Kintzios, S.E., Ed.; Taylor and Francis: London, UK, 2002; pp. 109–126. [Google Scholar]
- Altintas, A.; Tabanca, N.; Tyihák, E.; Ott, P.G.; Móricz, A.M.; Mincsovics, E.; Wedge, D.E. Characterization of volatile constituents from Origanum onites and their antifungal and antibacterial activity. J. AOAC Int. 2013, 96, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Ozek, T.; Tumen, G.; Sezik, E. Composition of the essential oils of Turkish Origanum species with commercial importance. J. Essent. Oil Res. 1993, 5, 619–623. [Google Scholar] [CrossRef]
- Tepe, B.; Cakir, A.; Tepe, A.S. Medicinal uses, phytochemistry, and pharmacology of Origanum onites (L.): A Review. Chem. Biodivers. 2016, 13, 504–520. [Google Scholar] [CrossRef] [PubMed]
- WHO Malaria Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 2 September 2019).
- WHO Human African Trypanosomiasis Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 2 September 2019).
- WHO American Trypanosomiasis Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 2 September 2019).
- WHO Leishmaniasis Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 2 September 2019).
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components—A resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef]
- Veal, L. The potential effectiveness of essential oils as a treatment for head lice, Pediculus humanus capitis. Complement. Ther. Nurs. Midwifery 1996, 2, 97–101. [Google Scholar] [CrossRef]
- Force, M.; Sparks, W.S.; Ronzio, R.A. Inhibition of enteric parasites by emulsified oil of Oregano in vivo. Phytother. Res. 2000, 14, 213–214. [Google Scholar] [CrossRef]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Dietary oregano essential oil supplementation on performance of broilers challenged with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef]
- Zimmermann, S.; Thomi, S.; Kaiser, M.; Hamburger, M.; Adams, M. Screening and HPLC-based activity profiling for new antiprotozoal leads from European plants. Sci. Pharm. 2012, 80, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Demirci, F.; Paper, D.H.; Franz, G.; Başer, K.H.C. Investigation of the Origanum onites L. essential oil using the chorioallantoic membrane (CAM) assay. J. Agric. Food Chem. 2004, 52, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, A.; Cook, C.M.; Lanaras, T.; Kokkini, S. The oregano plants of Chios Island (Greece): Essential oils of Origanum onites L. growing wild in different habitats. Ind. Crops Prod. 2016, 82, 107–113. [Google Scholar] [CrossRef]
- Özkan, G.; Baydar, H.; Erbas, S. The influence of harvest time on essential oil composition, phenolic constituents and antioxidant properties of Turkish oregano (Origanum onites L.). J. Sci. Food Agric. 2010, 90, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Kirmizibekmez, H.; Calis, I.; Perozzo, R.; Brun, R.; Dönmez, A.A.; Linden, A.; Rüedi, P.; Tasdemir, D. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodial enoyl-ACP reductase, a crucial enzyme in fatty acid biosynthesis. Planta Med. 2004, 70, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Kirmizibekmez, H.; Atay, I.; Kaiser, M.; Yesilada, E.; Tasdemir, D. In vitro antiprotozoal activity of extracts of five Turkish Lamiaceae species. Nat. Prod. Commun. 2011, 6, 1697–1700. [Google Scholar] [CrossRef] [Green Version]
- Atay, I.; Kirmizibekmez, H.; Kaiser, M.; Akaydin, G.; Yesilada, E.; Tasdemir, D. Evaluation of in vitro antiprotozoal activity of Ajuga laxmannii and its secondary metabolites. Pharm. Biol. 2016, 54, 1808–1814. [Google Scholar] [CrossRef] [Green Version]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; de Moura, R.O.; de Araújo, R.S.A.; Monteiro, K.L.C.; de Aquino, T.M.; Ribeiro, F.F.; et al. Active essential oils and their components in use against neglected diseases and arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [Green Version]
- Fujisaki, R.; Kamei, K.; Yamamura, M.; Nishiya, H.; Inouye, S.; Takahashi, M.; Abe, S. In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian Trop. Med. Public Health 2012, 43, 270–279. [Google Scholar]
- Milhau, G.; Valentin, A.; Benoit, F.; Mallie, M.; Bastide, J.-M.; Pelissier, Y.; Bessiere, J.-M. In vitro antimalarial activity of eight essential oils. J. Essent. Oil Res. 1997, 9, 329–333. [Google Scholar] [CrossRef]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011, 76, 512–518. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Viljoen, A.M. Pharmacological interactions of essential oil constituents on the in vitro growth of Plasmodium falciparum. S. Afr. J. Bot. 2010, 76, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.L.; Lobo, L.T.; Costa, J.M.; Costa, L.S.; Rocha, H.A.; Rocha e Silva, L.F.; Pohlit, A.M.; Neto, V.F. In vitro and in vivo antimalarial activity of essential oils and chemical components from three medicinal plants found in North eastern Brazil. Planta Med. 2012, 78, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Santoro, G.; Sousa, M.C.; Salgueiro, L.; Cavaleiro, C. Activity of essential oils on the growth of Leishmania infantum promastigotes. Flavour Fragr. J. 2010, 25, 156–160. [Google Scholar] [CrossRef]
- Teles, A.M.; Rosa, T.D.D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils: Chemical composition, antimicrobial and antileishmanial activity. Evid. Based Complement. Altern. Med. 2019, 2019, 2421695. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Dakka, N.; Talbaoui, A.; Et-Touys, A.; Bakri, Y. Correlation between phenological changes, chemical composition and biological activities of the essential oil from Moroccan endemic Oregano (Origanum compactum Benth). Ind. Crops Prod. 2017, 108, 729–737. [Google Scholar] [CrossRef]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Farias, P.A.; Rios, M.C.; Moura, T.A.; Almeida, R.P.; Alves, P.B.; Blank, A.F.; Fernandes, R.P.M.; Scher, R. Leishmanicidal activity of carvacrol-rich essential oil from Lippia sidoides Cham. Biol. Res. 2012, 45, 399–402. [Google Scholar] [CrossRef] [Green Version]
- De Melo, J.O.; Bitencourt, T.A.; Fachin, A.L.; Cruz, E.M.; de Jesus, H.C.; Alves, P.B.; de Fátima Arrigoni-Blank, M.; de Castro Franca, S.; Beleboni, R.O.; Fernandes, R.P.; et al. Antidermatophytic and antileishmanial activities of essential oils from Lippia gracilis Schauer genotypes. Acta Trop. 2013, 128, 110–115. [Google Scholar] [CrossRef]
- Xavier, F.J.S.; Rodrigues, K.A.F.; De Oliveira, R.G.; Lima Junior, C.G.; Rocha, J.D.C.; Keesen, T.S.L.; De Oliveira, M.R.; Silva, F.P.L.; Vasconcellos, M.L.A.A. Synthesis and in vitro anti-Leishmania amazonensis biological screening of Morita-Baylis-Hillman adducts prepared from eugenol, thymol and carvacrol. Molecules 2016, 21, 1483. [Google Scholar] [CrossRef] [Green Version]
- De Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.; Rondon, F.C.; Lobo, C.H.; de Alencar, A.N.M.A.; Sales, A.D.; Rodrigues, A.P.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [Green Version]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In vitro and in vivo effectiveness of carvacrol, thymol and linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, G.F.; das Graças Cardoso, M.; Guimarães, L.G.; Salgado, A.P.; Menna-Barreto, R.F.; Soares, M.J. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Escobar, P.; Leal, S.M.; Herrera, L.V.; Martinez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Cavadas, C.; Cavaleiro, C.; Salgueiro, L.; do Céu Sousa, M. In vitro susceptibility of Trypanosoma brucei brucei to selected essential oils and their major components. Exp. Parasitol. 2018, 190, 34–40. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef]
- Robledo, S.; Osorio, E.; Munoz, D.; Jaramillo, L.M.; Restrepo, A.; Arango, G.; Velez, I. In vitro and in vivo cytotoxicities and antileishmanial activities of thymol and hemisynthetic derivatives. Antimicrob. Agents Chemother. 2005, 49, 1652–1655. [Google Scholar] [CrossRef] [Green Version]
- Juan, R.A.; Olga, P.A.; Mirian, P.P. Chemical composition and anti-Trypanosoma cruzi effect of Thymus vulgaris L. (Thyme) essential oil and its main component, thymol, in mice. Am. J. Pharm. Pharmacol. 2015, 2, 21–27. [Google Scholar]
- Ozek, T.; Tabanca, N.; Demirci, F.; Wedge, D.E.; Baser, K.H.C. Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod. 2010, 4, 180–192. [Google Scholar]
- Mikus, J.; Harkenthal, M.; Steverding, D.; Reichling, J. In vitro effect of essential oils and isolated mono- and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 2000, 66, 366–368. [Google Scholar] [CrossRef]
- Leal, S.M.; Pino, N.; Stashenko, E.E.; Martinez, J.R.; Escobar, P. Antiprotozoal activity of essential oils derived from Piper spp. grown in Colombia. J. Essent. Oil Res. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Grando, T.H.; Souza, C.F.; Gressler, L.T.; Stefani, L.M.; da Silva, A.S.; Monteiro, S.G. In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasitol. 2016, 162, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Pina-Vaz, C.; Rodrigues, A.G.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Goncalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.G.; Silva, A.C.; Cito, A.M.; Borges, A.R.; Lima, S.G.; Lopes, J.A.; Figueiredo, R.C. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Ogungbe, I.V.; Setzer, W.N. In-silico Leishmania target selectivity of antiparasitic terpenoids. Molecules 2013, 18, 7761–7847. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.C.; Jha, A.; Kumar, A.; Samant, M. Evaluation of antileishmanial potential of computationally screened compounds targeting DEAD-box RNA helicase of Leishmania donovani. Int. J. Biol. Macromol. 2019, 121, 480–487. [Google Scholar] [CrossRef]
- Guardo, N.I.; Sainz, P.; Gonzalez-Coloma, A.; Burillo, J.; Martinez-Diaz, R.A. Trypanocidal effects of essential oils from selected medicinal plants. Synergy among the main components. Nat. Prod. Commun. 2017, 12, 709–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enan, E. Synergistic compositions comprising two or more compounds selected from: Trans-anethole, p-cymene, linalool, α-pinene, and thymol, and methods for treating parasitic infections. PCT Int. Appl. WO 2008003007 A2 20080103, 24 December 2008. [Google Scholar]
- Gößling, A. Wirkungen eines Oreganoöl-Zusatzes als Futteradditiv auf die Darmflora von Absatzferkeln. Ph.D. Thesis, Tieraerztliche Hochschule, Hannover, Germany, 2001. [Google Scholar]
- Möller, T. Untersuchungen zum Einflußeines Oreganoöl-Zusatzes zum Futter auf die Rohnaehrstoffverdaulichkeit; N-Bilanz sowie auf Parameter des mikrobiellen Stoffwechsels im Verdauungstrakt von Absetzferkeln. Ph.D. Thesis, Tieraerztliche Hochschule, Hannover, Germany, 2001. [Google Scholar]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gong, J.; Huan, X.; Yu, H.; Xue, F. In vitro evaluation of the activity of microencapsulated carvacrol against Escherichia coli with K88 pili. J. Appl. Microbiol. 2009, 107, 1781–1788. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Duman, G.; Corapcıoğlu, G.; Baranauskas, A.; Taralp, A.; Ivanauskas, L.; Bernatoniene, J. Liposomal incorporation to improve dissolution and stability of rosmarinic acid and carvacrol extracted from Oregano (O. onites L.). BioMed Res. Int. 2018, 2018, 6147315. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; Beirão da Costa, S.; Beirão da Costa, L.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Pan, K.; Chen, H.; Davidson, P.M.; Zhong, Q. Thymol nanoencapsulated by sodium caseinate: Physical and antilisterial properties. J. Agric. Food Chem. 2014, 62, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Baranauskaite, J.; Kopustinskiene, D.M.; Bernatoniene, J. Impact of gelatin supplemented with gum arabic, tween 20, and β-cyclodextrin on the microencapsulation of Turkish oregano extract. Molecules 2019, 24, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the foodborne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; John Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Hochmuth, D.H. MassFinder 4.0; Hochmuth Scientific Consulting: Hamburg, Germany, 2008. [Google Scholar]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Matile, H.; Pink, J.R.L. Plasmodium falciparum malaria parasite cultures and their use in immunology. In Immunological Methods; Lefkovits, I., Pernis, B., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Thaithong, S.; Beale, G.H.; Chutmongkonkul, M. Susceptibility of Plasmodium falciparum to five drugs: An in vitro study of isolates mainly from Thailand. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 228–231. [Google Scholar] [CrossRef]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar] [CrossRef]
- Räz, B.; Iten, M.; Grether-Buhler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J. Protozool. 1977, 24, 325–329. [Google Scholar] [CrossRef]
- Page, C.; Page, M.; Noel, C. A new fluorimetric assay for cytotoxicity measurements in vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [PubMed]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immun. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the authors. |

| No | RRI a | Compound | % | Identification Method b |
|---|---|---|---|---|
| 1 | 1018 | Methyl 2-methyl-butyrate | 0.1 | MS |
| 2 | 1024 | Methyl 3-methyl-butyrate | tr c | MS |
| 3 | 1032 | α-Pinene | 0.5 | RRI, MS |
| 4 | 1035 | α-Thujene | 0.1 | RRI, MS |
| 5 | 1076 | Camphene | 0.2 | RRI, MS |
| 6 | 1118 | β-Pinene | tr | RRI, MS |
| 7 | 1159 | δ-3-Carene | 0.1 | MS |
| 8 | 1174 | Myrcene | 1.0 | RRI, MS |
| 9 | 1188 | α-Terpinene | 1.0 | RRI, MS |
| 10 | 1203 | Limonene | 0.2 | RRI, MS |
| 11 | 1213 | 1,8-Cineole | 0.3 | RRI, MS |
| 12 | 1218 | β-Phellandrene | 0.2 | RRI, MS |
| 13 | 1246 | (Z)- β-Ocimene | 0.1 | MS |
| 14 | 1255 | γ-Terpinene | 2.1 | RRI, MS |
| 15 | 1280 | p-Cymene | 7.0 | RRI, MS |
| 16 | 1290 | Terpinolene | 0.1 | RRI, MS |
| 17 | 1393 | 3-Octanol | 0.1 | MS |
| 18 | 1450 | trans-Linalool oxide (Furanoid) | tr | MS |
| 19 | 1452 | α,p-Dimethylstyrene | tr | MS |
| 20 | 1452 | 1-Octen-3-ol | 0.3 | MS |
| 21 | 1474 | trans-Sabinene hydrate | 0.4 | MS |
| 22 | 1478 | cis-Linalool oxide (Furanoid) | 0.1 | MS |
| 23 | 1497 | α-Copaene | tr | MS |
| 24 | 1532 | Camphor | tr | RRI, MS |
| 25 | 1553 | Linalool | 9.7 | RRI, MS |
| 26 | 1571 | trans-p-Menth-2-en-1-ol | tr | MS |
| 27 | 1610 | Calarene (=β-gurjunene) | tr | MS |
| 28 | 1611 | Terpinen-4-ol | 0.7 | RRI, MS |
| 29 | 1612 | β-Caryophyllene | 0.7 | RRI, MS |
| 30 | 1620 | Selina-5,11-diene | tr | MS |
| 31 | 1628 | Aromadendrene | 0.2 | MS |
| 32 | 1638 | cis-p-Menth-2-en-1-ol | tr | MS |
| 33 | 1645 | cis-Isodihydrocarvone | tr | MS |
| 34 | 1661 | Alloaromadendrene | tr | MS |
| 35 | 1670 | trans-Pinocarveol | tr | RRI, MS |
| 36 | 1682 | δ-Terpineol | tr | MS |
| 37 | 1687 | α-Humulene | tr | RRI, MS |
| 38 | 1689 | trans-Piperitol (=trans-p-Menth-1-en-3-ol) | tr | MS |
| 39 | 1704 | γ-Muurolene | tr | MS |
| 40 | 1706 | α-Terpineol | 0.7 | RRI, MS |
| 41 | 1708 | Ledene | 0.1 | MS |
| 42 | 1719 | Borneol | 0.5 | RRI, MS |
| 43 | 1740 | α-Muurolene | tr | MS |
| 44 | 1751 | Carvone | 0.1 | RRI, MS |
| 45 | 1773 | δ-Cadinene | 0.1 | MS |
| 46 | 1776 | γ-Cadinene | tr | MS |
| 47 | 1798 | Methyl salicylate | tr | RRI, MS |
| 48 | 1845 | trans-Carveol | 0.1 | MS |
| 49 | 1864 | p-Cymen-8-ol | 0.1 | RRI, MS |
| 50 | 1940 | 4-Isopropyl salicylaldehyde | 0.1 | MS |
| 51 | 1983 | Piperitenone oxide | tr | RRI, MS |
| 52 | 2008 | Caryophyllene oxide | 0.3 | RRI, MS |
| 53 | 2030 | Methyl eugenol | tr | RRI, MS |
| 54 | 2033 | Epiglobulol | tr | MS |
| 55 | 2050 | (E)-Nerolidol | tr | MS |
| 56 | 2071 | Humulene epoxide-II | tr | MS |
| 57 | 2098 | Globulol | tr | MS |
| 58 | 2104 | Viridiflorol | tr | MS |
| 59 | 2113 | Cumin alcohol | tr | RRI, MS |
| 60 | 2144 | Spathulenol | 0.1 | MS |
| 61 | 2181 | Isothymol (=2-Isopropyl-4-methyl phenol) | tr | MS |
| 62 | 2185 | γ-Eudesmol | tr | MS |
| 63 | 2186 | Eugenol | tr | RRI, MS |
| 64 | 2198 | Thymol | 1.8 | RRI, MS |
| 65 | 2221 | Isocarvacrol (=4-Isopropyl-2-methyl phenol) | tr | MS |
| 66 | 2239 | Carvacrol | 70.6 | RRI, MS |
| 67 | 2246 | 3-Isopropyl-2-methyl phenol | 0.1 | MS |
| 68 | 2250 | α-Eudesmol | tr | MS |
| 69 | 2257 | β-Eudesmol | tr | MS |
| 70 | 2300 | 3-Isopropyl-5-methyl phenol | tr | MS |
| 71 | 2392 | Caryophylla-2(12),6-dien-5β-ol (=Caryophyllenol II) | tr | MS |
| Monoterpene hydrocarbons | 12.9 | |||
| Oxygenated monoterpenes | 84.9 | |||
| Sesquiterpene hydrocarbons | 1.1 | |||
| Oxygenated sesquiterpenes | 0.4 | |||
| Diterpenes | 0.6 | |||
| Others | 12.9 | |||
| Total | 99.9 |
| Sample | Trypanosoma brucei | Trypanosoma | Leishmania | Plasmodium | Cytotoxicity |
|---|---|---|---|---|---|
| rhodesiense | cruzi | donovani | falciparum | L6 cells | |
| Standard compd | 0.003 a | 0.44 b | 0.2 c | 0.056 d | 0.004 e |
| O. onites Essential Oil | 0.18 ± 0.004 | >90 | 17.8 ± 3.7 | 7.9 ± 0.3 | >90 |
| Carvacrol | 0.15 ± 0.04 | >90 | 13.1 ± 3.9 | 6.4 ± 0.9 | 48.8 ± 1.1 |
| Thymol | 0.11 ± 0.01 | >90 | 17.3 ± 4.1 | 5.7 ± 0.03 | 51.8 ± 6.2 |
| α-Pinene | 0.42 ± 0.24 | >90 | 81.9 ± 9 | 10.7 ± 1.2 | 87.8 ± 3 |
| Myrcene | 22 ± 14.8 | >90 | 48.2 ± 12.7 | >20 | >90 |
| α-Terpinene | 3.1 ± 1.6 | 49.1 ± 7.3 | 10.5 ± 1.7 | 3.7 ± 1.5 | 84.7 ± 4.3 |
| γ-Terpinene | 32.9 ± 26 | >90 | >90 | >20 | >90 |
| p-Cymene | 45.0 ± 27 | >90 | >90 | >20 | >90 |
| L-Linalool | 3.6 ± 2.5 | >90 | 86.3 ± 2.8 | >20 | >90 |
| Terpinen-4-ol | 0.66 ± 0.4 | 46.8 ± 7.4 | 68.7 ± 14.2 | >20 | 43.3 ± 16.5 |
| β-Caryophyllene | 28.9 ± 11.8 | 50.1 ± 12.5 | 52.4 ± 17.4 | 12.8 | 62.2 ± 7 |
| α-Terpineol | 0.56 ± 0.4 | 61.0 ± 2.1 | 75.9 ± 4.7 | >20 | 32.3 ± 0.1 |
| Borneol | 24.3 ± 11.8 | >90 | 52.1 ± 16.6 | >20 | >90 |
| Carvacrol Methyl Ether | 17.0 ± 22 | >90 | 17.5 ± 1.7 | >20 | >90 |
| Thymol Methyl Ether | 4.1 ± 1.97 | >90 | 86.0 ± 1.9 | >20 | >90 |
| Thymoquinone | 0.11 ± 0.02 | 18.2 ± 4.3 | 1.7 ± 0.16 | 2.6 ± 0.16 | 20.5 ± 8.1 |
| Compound | Dosing Regimen (mg/kg) × 4 days | Injection | Cured/Infected | Mean Survival (days) |
|---|---|---|---|---|
| Essential oil | 100 | i.p. | 0/4 | 5.25 |
| Thymol | 100 | i.p. | 0/4 | 9.0 |
| Carvacrol | 100 | i.p. | 0/4 | 5.25 |
| Control a | - | - | 0/4 | 5.25 |
| Standard b | 5.0 | i.p. | 4/4 | >30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tasdemir, D.; Kaiser, M.; Demirci, B.; Demirci, F.; Baser, K.H.C. Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components. Molecules 2019, 24, 4421. https://doi.org/10.3390/molecules24234421
Tasdemir D, Kaiser M, Demirci B, Demirci F, Baser KHC. Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components. Molecules. 2019; 24(23):4421. https://doi.org/10.3390/molecules24234421
Chicago/Turabian StyleTasdemir, Deniz, Marcel Kaiser, Betül Demirci, Fatih Demirci, and K. Hüsnü Can Baser. 2019. "Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components" Molecules 24, no. 23: 4421. https://doi.org/10.3390/molecules24234421
APA StyleTasdemir, D., Kaiser, M., Demirci, B., Demirci, F., & Baser, K. H. C. (2019). Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components. Molecules, 24(23), 4421. https://doi.org/10.3390/molecules24234421